
When we first caught up with Verisante (TSXV:VRS) CEO Thomas Braun, the company was adding engineering staff to further the develompment of Aura, its skin cancer detection device.
Today, Verisante is embarking on a controlled rollout of the device, after gathering valuable data from a limited release to a half-dozens clinics. 2014 will be a big year for the company, as it will see the first stage of a marketing campaign in Europe and Canada, and an FDA clinical trial in the U.S.
Verisante also has a two new products.Core, an endoscopic version of Aura, has completed a successful study, with the BC Cancer Agency, whom Verisante has deep ties to. And a futuristic new multispectral imaging camera for skin and oral cancer the company has developed is a small hand held device that lends itself to being paired to smart phones and tablets.
Cantech Letter caught up with Verisante CEO Thomas Braun to talk about how these products might be a game-changer in the early detection of various forms of Cancer.
Thomas, can you tell me about the major events at Verisante since we last caught up with you?
You already know about the Popular Science Best of What’s New award we received for Aura; the Canadian Cancer Society naming the Core one of the Top Ten Cancer Breakthroughs; the TSXV Pick of the Street Award; and the TSXV Top Technology and Life Science Company for 2012. In 2013 we won the Prism Award for the Aura which is given out at the biggest optics and photonics conference in the world held in San Francisco. I had the honor of accepting the award at a gala event with the inventing team from the BC Cancer Agency. What made it really special was that we beat world class companies who were finalists for the award including Olympus. We also won an Edison Award for Innovation which was also a great honor for us.
Sales of the Aura started around April, 2013. How has that progressed?
We learned that the role-out of a highly sophisticated medical device has to be carried out in stages. We started with a limited market release of Aura by placing it in six clinics. Over the subsequent months we collected valuable feedback from doctors. We also had a chance to see how our training, manuals and user interface worked in the real world. We also gained experience in setting up our field service, logistics, training, and customer relations.
We decided to upgrade our user interface and training for operators in response to some of the feedback we received. We also made some changes to make the device more rugged for shipping. This limited release in Canada is the first stage of a full marketing campaign in Canada and Europe; and an FDA clinical trial in the United States.
I feel confident that we are making the right decisions, and by taking a cautious approach through a controlled roll out, we have avoided potential problems down the road. Now that we have this initial stage behind us we will be well positioned to increase our sales and marketing efforts in 2014.
Another thing that has happened since our release to a handful of clinics has been the beginning of sales into the research market. Companies like Thermo Fisher and Brucker Optics make billions in this market. We feel we have a very strong product offering with the research version of Aura, which is essentially the same device and probe, but without the diagnostic algorithm for skin cancer. Our device is the only Raman spectrometer that we know of that can take instant measurements on live patients. So any kind of skin research on diseases, drugs, or cosmetics that you can think of could benefit from being able to non-invasively measure the changing biochemistry of the skin. We have already sold several devices into the research market. It’s also worth noting that not too long ago Thermo Fisher bought a Raman spectrometer startup company called Ahura Scientific for $165 million.
As you know, in addition to the skin probe we also have a fibre-optic probe for endoscopic applications. Besides medical research, fiber-optic probes can be easily adapted to various applications such as measuring biological samples, material sciences, forensics, pharmaceutical science, nanotechnology and archaeology. The important message about the research units is that they can be sold worldwide in every market without needing regulatory approvals from health authorities. This can translate into immediate short term revenue before we are even approved by the FDA, China, Japan, etc.
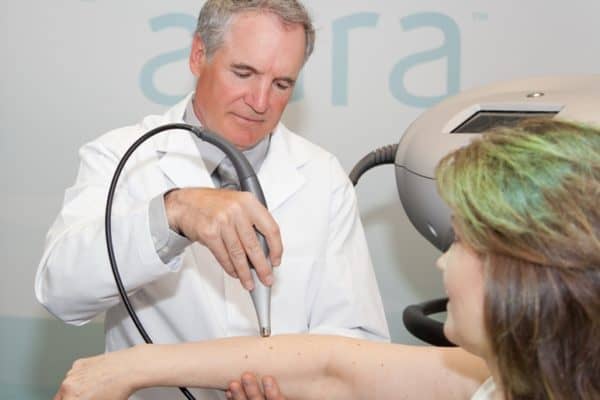
In February, Verisante won a 2013 Prism Award in the Life Sciences and Biophotonics category for its Aura™ skin cancer detection device.
Aura is approved in Canada and Europe. What about US FDA approval?
I think it’s obvious that the United States will be the main market for Aura. The difference is that they have private healthcare and since they do not have the same shortage of doctors that we have in Canada, their doctors and clinics actually have to compete for patients. One major way to differentiate yourself from the competition is to offer superior technology. American dermatologists typically advertise the devices they have and are quicker to utilize the latest technology to stay ahead of the curve.
We have been preparing the initial submission to the FDA and we anticipate that will go ahead in the next several weeks. I think that the full sales rollout of Aura in Canada and Europe, and our first filing with the FDA will be major milestones for us. The clinical study for the US should take about a year and then it usually takes twelve to eighteen months to process a PMA application.
The last time we spoke, the endoscopic version of Aura, which is called the Core, was being used in a lung cancer study at the BC Cancer Agency. How is the clinical study for the Core progressing?
The lung cancer study at the BC Cancer Agency which started about two years ago is done. They used the rapid Raman system, which we branded Core, on about three hundred patients. The technology is the same as used in Aura for skin cancer detection except that the probe is a very thin fiber optic probe that goes down the working channel of the endoscope. The pilot study results were published some time ago in a high impact journal and were really amazing. We are now looking forward to the final analysis of the results, which are expected in the next few weeks. I think that will be a major milestone for both Verisante and the BC Cancer Agency because lung cancer was always the end game of the BC Cancer Agency and funding agencies like the Canadian Cancer Society and the Canadian Institutes for Health Research, as it is by far the biggest cancer killer. There are about 1.4 million people dying of lung cancer each year. The potential impact of this technology is huge because the key to surviving lung cancer is early detection. If you detect lung cancer in stage one you have a 90% chance of survival, but that drops to 1% in stage four.
I don’t think many people realize that the Core device works across many different surgical specialties for cancer detection which has been the key driver behind the success of products like Novadaq’s SPIE imaging system. Besides lung cancer, there is a colon cancer study just starting at the BC Cancer Agency. Colon cancer is the second biggest cancer killer. A study on nasalpharyngeal cancer has just begun in China at the Fujian Provincial Tumor Hospital which is a key medical research centre. Nasalpharyngeal cancer is quite common in Southern China and we are thrilled to be in collaboration with the BC Cancer Agency and this prestigious research centre in China, which is undoubtedly a massive market for the Core. There have also been clinical studies on esophageal and stomach cancer that used our Core technology in Singapore on about five hundred patients with excellent results. I think the studies in Singapore and China reflect the strong demand for this kind of technology in Asia.
All of these studies have been fully funded by research grants which are also a reflection of the quality and the anticipated impact of the technology. Not only do our investors get the benefit of ten years of development by the BC Cancer Agency, but they also benefit from these numerous studies which have all been supported by peer reviewed grants. As the results are published for each of these clinical studies we can expand the uses of this device and announce new milestone achievements. As you can see there are quite a few milestone achievements in the pipeline and we are currently organizing more studies.
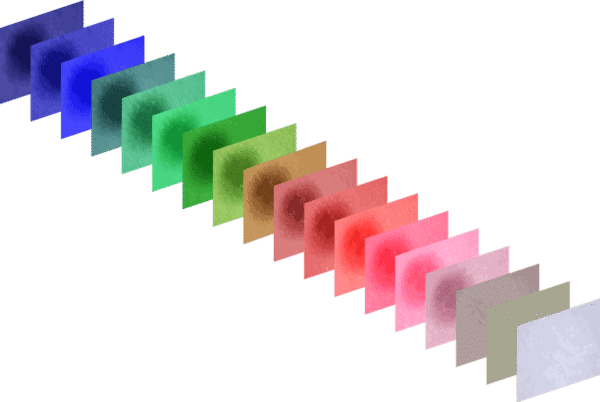
Can you tell us about the development schedule of the Core?
We expect to have a commercial prototype built by the end of this year. This will be submitted for safety testing which can take several months.
Then once we have the final statistical analysis from the BC Cancer Agency we can apply for Health Canada approval and a CE Mark. This will speed up the process of applying for regulatory approval in other countries such as China and Japan. To start with, doctors in these other countries can do clinical studies where we only supply the device with no additional cost to the company similar to what is being done with some of our current studies. Full regulatory approval in these other countries typically takes two years, but the reward could be substantial. In China alone there are over 21,000 hospitals and each year China has four to five million new cases of lung cancer.
After we have obtained Health Canada Approval and a CE Mark for Core we can move forward with the FDA approval process which usually takes about two years.
One last thing I want to add about the Core is that since it works with every kind of endoscope we have come across that is used to detect and treat different cancers from colon to brain; it is a very valuable accessory to any endoscopic surgical system. I think this makes Core a very attractive potential strategic partnership candidate for the majors many of which are cash rich and pipeline poor. The Core could give them multiple shots on goal and is a technology platform that a multimillion dollar business can be built on.
You recently issued a press release on a new product called a rapid multispectral imaging camera. Can you elaborate on what the product is for and how it will be positioned in the market vis a vis the Aura?
We announced two years ago that we were starting a project to build a multispectral imaging camera for skin and oral cancer after we had licensed the exclusive worldwide rights from the inventors at the BC Cancer Agency. Around that time we were also invited to meet with engineers from a major smart phone manufacturer who asked if we could adapt our cancer detection technology to work with their hand held products. While the Aura is a major piece of capital equipment, the MSI Camera is a small hand held device which lends itself to being paired to smart phones and tablets.
The MSI Camera takes 18 images in one third of a second, each using a different narrow band wave length. Each of these wave lengths allows us to measure a different parameter such as the oxygenation of hemoglobin, melanin content, etc., and these measurements together can be used to determine if the tissue is malignant or benign.
We recently announced an advanced stage prototype and showed pictures on our Facebook page. The device is currently being laboratory tested at the BC Cancer Agency.
For skin cancer we will be competing with digital dermatoscopes which are hand held digital cameras with magnifying lenses. They can cost anywhere from a few hundred dollars to several thousand. They only have a single wave length and do not have diagnostic algorithms, so we will have a tremendous competitive advantage since our device will do everything the competition does and more. We plan to sell the MSI Camera as a low cost alternative to Aura and also as an accessory to Aura itself. Since the MSI camera measures different parameters we believe that when we combine the two devices that the overall accuracy will improve. The MSI Camera will also take true colour dermatoscopic images for those who want a photographic image of the lesion in addition to an Aura scan.
As a standalone device we will be targeting family doctors and dermatologists. The pairing of the device via Wi-Fi with popular smart phone and tablets will help drive sales. We may also develop a consumer model for the mass market for personal use.
I am very excited about the oral cancer application because the competition is using twenty year old technology and there is already a billing code and reimbursement in the US for using any adjunctive device while screening for oral cancer. This means that a dentist can bill an extra $35 for using the device and we estimate that they can add about $20,000 to their annual revenue. This has driven sales of the devices that are currently on the market. There are only three competing devices that we know of and they are all relying on using one to three wavelengths to produce autofluorescence which causes all abnormal tissue to stand out. The problem with these old devices is that they are based on technology that was patented in the early nineties and is now in the public domain, so I anticipate many inexpensive imitators to start arriving on the market. The other problem with them is that they are very simple with only one to three different wave lengths, no built in camera, and no diagnostic algorithm. In fact, studies have shown that fluorescent illumination by itself is associated with a high level of false positives which is a serious drawback for oral cancer screening. The reason some dentists, nevertheless, use the old style devices now is because they have no alternatives. We intend to dominate this market, and with 170,000 dentists in the United States alone, the total market is potentially worth a few hundred million.
The device will follow the same regulatory pathway as all of our other devices. We will conduct a clinical study and collect data for skin cancer first and then the Oral cancer version will be next.
How do you plan to fund the development of all of these products?
Since inception we have raised over $18 million in capital and we will continue to raise funding in the capital markets as required, but we are also reaching out to bigger companies to form strategic partnerships for non-dilutive financing. Most of the development work is already done and many of the clinical studies are funded by granting agencies in various countries. With so many clinical studies already done or in progress, and the numerous awards we have won including two in 2013, we feel that now is the right time to start the process of partnering with other companies to assist in the regulatory approval, marketing and distribution of our product pipeline. We have reached out to many companies and the conversations are just beginning. Because sometimes these strategic partnerships are the first step towards a complete takeover, I think any announcement in this area could be a major catalytic event for our stock.
Click here for a recent video interview with Thomas Braun.
Disclosure: Verisante is an annual sponsor of Cantech Letter.
_________________
Leave a Reply
You must be logged in to post a comment.





 Share
Share Tweet
Tweet Share
Share




Comment