
The paper was authored, in part, by Dr. Haishan Zeng, who is part of Verisante’s product development team, and Dr. Harvey Lui, one of the inventors of Aura. Lui sits on Verisante’s board and is also the director of the Skin Care Centre at Vancouver General Hospital and Head of the Department of Dermatology and Skin Science at The University of British Columbia.
In a press release today, Lui offered a sneak peek at what the report will reveal. “This study clearly demonstrates that the Verisante Aura has significant diagnostic accuracy in distinguishing malignant from benign skin lesions..” he said, adding that the device “…offers the potential for reducing unnecessary biopsies by 50 per cent to 100 per cent. ”
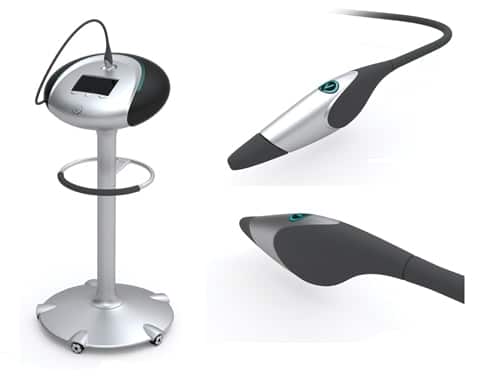
Verisante believes Aura, a non-invasive imaging and spectroscopy system, offers a more reliable way to diagnose skin cancer, which is currently done by visual examination, followed by a biopsy. The company says the accuracy of clinicians in correctly diagnosing skin cancer varies widely and that Aura offers a standardized approach that boasts a statistically significant improvement in the detection of skin cancer. Aura will allow doctors to scan up to forty skin lesions in a single session, and does not require specialized knowledge to interpret the results.
Verisante has received approval to sell Aura in Australia, Europe and here in Canada, where it will be distributed by Clarion Medical Technologies. The company intends to send several functioning prototypes to leading skin cancer clinics in the UK, Australia and the US and to begin generating revenue later this year, while gathering data with an eye towards FDA approval.
At press time, shares of Verisante were up 1.6% to $.63 cents.
Leave a Reply
You must be logged in to post a comment.







 Share
Share Tweet
Tweet Share
Share




Comment