
 Editors Note: Canada’s medical marijuana industry is undergoing a sea-change. One need look no further than the public markets for evidence; the country’s first publicly traded marijuana play, Tweed Marijuana, recently began trading and boasts a market cap in excess of $100-million.
Editors Note: Canada’s medical marijuana industry is undergoing a sea-change. One need look no further than the public markets for evidence; the country’s first publicly traded marijuana play, Tweed Marijuana, recently began trading and boasts a market cap in excess of $100-million.
New regulations have altered the landscape on the supply side and will treat marijuana more like a medication. This will effectively alter the industry forever. The changes will create a new space that is not without risks, but will result in many high-margin businesses in a sector that should boast strong double-digit growth for years to come.
M Partners has graciously allowed us to share a report with you from analyst Daniel Pearlstein that represents what we feel is the most current and comprehensive look at the nascent industry.
INDUSTRY INSIGHT: MEDICAL MARIJUANA
Canadian Regulatory Reform Brings New Opportunity
OVERVIEW
-Regulatory changes to the medical marijuana (“MMJ”) industry in Canada have shifted the landscape of the supply chain
-The old system allowed self production of MMJ that caused regulatory, safety, and trafficking concerns for Health Canada
-The new system licenses a small number of commercial producers to grow, produce, distribute, and sell
-A highly regulated industry should more effectively handle expected growth in demand from a growing qualified patient population
-There are approximately 40,000 qualified patients in Canada today; this number is expected to grow to over 450,000 in 10 years (source: Health Canada)
-Only 13% of qualified patients used the (formerly) one and only Health Canada contracted producer; the remaining 87% will now need to engage newly licensed producers starting April 1, 2014 (source: Health Canada)
-Early licensees have a first mover advantage for customer capture and lock-in as they increase their brand recognition

QUICK HISTORY LESSON
Qualified patients in Canada were first granted the authority to possess marijuana for medical purposes in 1999 under the Marijuana Medical Access Program (MMAP). Due to issues encountered by those who needed MMJ, particularly the lack of legal sources, the Marijuana Medical Access Regulations (MMAR) was established in 2001 to allow qualified patients to possess MMJ with the support of a medical practitioner. Further amendments were made to the MMAR over the next ten years to alter the supply and distribution scheme including those that aimed to provide reasonable access to legal supply, streamline regulatory requirements and applications, and increase the number of licenses a producer can hold. Cannabis is still not an approved drug or medicine in Canada. Possession and use remains illegal unless authorized under regulations with the support of a healthcare practitioner for the relief of pain and other indications.
THE OLD SYSTEM: MARIJUANA MEDICAL ACCESS REGULATIONS (MMAR)
There were three ways to access medical marijuana under the old system:
1) Produce your own – under a Personal Use Production License (PUPL) [64% of qualified patients]
2) Designate a grower – under a Designated Person Production License (DPPL) [16% of qualified patients]
3) Purchase from Health Canada – via a contracted private company [13% of qualified patients]
According to Health Canada, 93% of patients acquired MMJ by any of these three methods with the remaining 7% of patients acquiring it somehow else. Notably, only 13% of patients acquired MMJ through Health Canada. Concerns of public health, safety, and security were raised since the vast majority of qualified patients produced marijuana under PUPLs and DPPLs within private residences. Abuse of the allotted licenses, exposure to chemicals and electrical hazards, and most obviously, diversion were all concerns raised by Health Canada, municipalities, and first responders such as police and fire officials. Complaints were also raised by patients regarding the limited number of strains offered and the long application processing times.
The combination of the above concerns and the growing administration costs incurred by Health Canada swayed the regulatory body to find a new solution. The MMAR was repealed on March 31, 2014 to give way to the new regulatory system – all authorizations and licenses issued under the MMAR would be no longer valid, and all plants and seeds used for production were ordered to be destroyed.

THE NEW SYSTEM: MARIJUANA FOR MEDICAL PURPOSES REGULATIONS (MMPR)
The MMPR aims to treat marijuana as much like a medication as possible by creating a licensing scheme for the commercial production and distribution of marijuana for medical purposes. The MMPR outlines strict and standardized policies for growing, manufacturing, safe keeping, security, record keeping (for at least two years), packaging, labeling, and shipping MMJ. The new regulations provide:
– For licensed producers, a structured and regulated guideline
– For qualified patients, increased choice of marijuana strains and suppliers
– For Health Canada, reduced role and costs of administering MMJ
-For personal growers and law enforcement, reduced safety concerns
A patient “who obtain[s] support from their health care practitioner by means of a medical document under the new system can only obtain their supply of dried marijuana from a licensed producer” [Transitioning to the New System, Health Canada]. The process from a patient perspective (simplified):
– The patient receives consent from a health care practitioner in the form of a medical document
– The patient registers with a licensed producer and provides medical documentation and shipping information
– The medical documents are verified by the licensed producer, and confirmation is sent to the patient
– The patient can then order MMJ from the licensed producer within a 30 day period up to the lesser of 150 grams or 30 times the daily quantity of prescribed MMJ
– The patient may obtain MMJ from more than once source at a time only if their healthcare practitioner grants multiple medical documents (e.g. one document for 15 grams from one producer, or three documents for 5 grams from three different producers)
Under MMPR, the healthcare practitioner is not only authorized to provide the medical document, but also to sell, provide, or administer MMJ. The healthcare practitioner must specify the period of use and the validity of the medical document. Practicing physicians may show resistance to holding onto large quantities of MMJ in their offices but registered nurses that provide care and/or visits to multiple patients may be more inclined to administration.

CANNABIS, TETRAHYDROCANNABINOL (THC), AND CANNABIDIOL (CBD)
The cannabis plant, aka cannabis sativa, contains more than 80 cannabinoids, a group of chemical compounds which includes delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Research has shown that THC and CBD influence different regions of the central nervous system and have different effects on cannabis users [Borgwardt, Biol Psychiatry, 2008]. Most of the psychoactive effects associated with the use of cannabis are caused by THC, whereas CBD has been shown to have anti-anxiety, anti-nausea, anti-inflammatory, and anti-psychotic effects [Bergamaschi, Curr Drug Saf., 2011; Niesink, Front Psychiatry, 2013]. Cannabis smoking often leads to adverse effects such as increases and fluctuations in heart rate and blood pressure, euphoria, anxiety, and impairment of cognition and memory. Cannabis also contains a similar array of detrimental and carcinogenic compounds compared to cigarette smoke, some of which are present even at higher concentrations [Leung, J Am Board Fam Med, 2011].
MMJ IS USED IN A WIDE VARIETY OF DISEASES
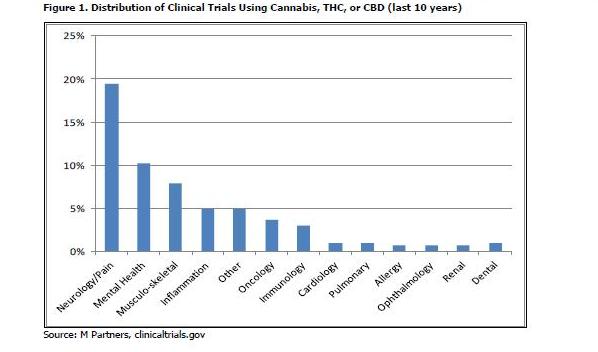
MMJ is used and has been tested in a variety of indications. In a search for clinical trials started in the last ten years, we found over 300 individually registered trials used cannabis, THC, or CBD as the intervention. Excluding addiction, the indication that accounted for the majority (42%) of trials, we found that MMJ has been tested in a wide range of indications to help patients cope with pain not only from the disease itself, but also for relief from strong and sometimes toxic medication, such as chemotherapy. Neurological disorders, mental health, muscle and back problems, and inflammation (such as gastrointestinal disorders) were most prevalent in this list.
SOME PRODUCTS ON THE MARKET
The use of cannabis, THC, or CBD in medicine is not completely new and there are a handful of drugs currently on the market that contain them. GW Pharmaceuticals’ (NASDAQ:GWPH | N/R) Sativex® is used for the treatment of spasticity due to multiple sclerosis, and is also in development to address cancer pain and neuropathic pain. Valeant Pharmaceuticals’ (VRX:NASDAQ | N/R) Nabilone / Cesamet® is also used for nausea and vomiting in patients undergoing cancer treatment. Abbott (NYSE:ABT | N/R) subsidiary Solvay Pharmaceuticals’ Dronabinol / Marinol® is used for nausea and vomiting for patients undergoing cancer treatment and neuropathic pain in patients with multiple sclerosis.
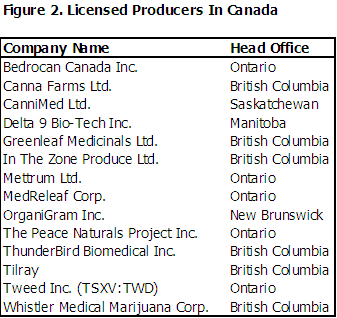
LICENSED PRODUCERS ARE ALREADY IN PRODUCTION
Health Canada has issued 14 licenses to commercial producers as of the published date of this report. Six are located in British Columbia, five are located in Ontario, and one each in Manitoba, New Brunswick, and Saskatchewan.
Companies in the space are expected to have high margins as prices seem to initially be between $5-$10 per gram while COGS could be only $1-$2 per gram. The bulk of expenses are likely to be for capital expenditures, production, sales and marketing, and security. Attractive companies will be those that can show the ability to manufacture, deliver, and scale quickly. Producers should have experienced cultivation and horticulture specialists, careful quality control and security measures, and meticulous record-keeping procedures.

INDUSTRY METRICS
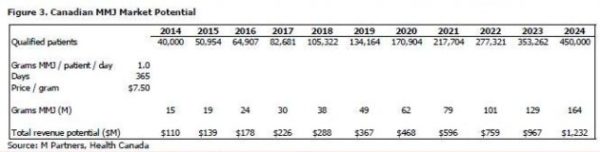
In 2002, there were less than 500 individuals in Canada authorized to posses MMJ; by 2012 this number grew to almost 22,000. Health Canada estimates that there are currently 40,000 qualified patients in Canada and that patient population is expected to grow to 450,000 over the next ten years (27.4% CAGR), which based on current general population growth rates would represent over 1% of the Canadian population in 2024. Drivers for growth on top of regulatory reform include the growing elderly population, the changing paradigm regarding the use of MMJ for the relief of pain and other indications, and participation from healthcare practitioners.
Prices under the old regulation ranged from $2-$5 per gram, whereas under MMPR Health Canada assumes licensed producers could charge $7-$10 per gram. It should be noted that Health Canada has no influence on the pricing set by licensed producers.
Research in the Netherlands [Hazekamp, Eur. J. Clin. Pharmacol., 2013] tracked over 5,000 qualified patients using MMJ from 2003-2010 and found the average daily amount of MMJ used was 0.68 grams per day (range: 0.65 – 0.82 grams per day). Health Canada also quotes data from Israel’s medical marihuana program that the average daily amount used by patients was approximately 1.5 grams per day in 2011-2012.
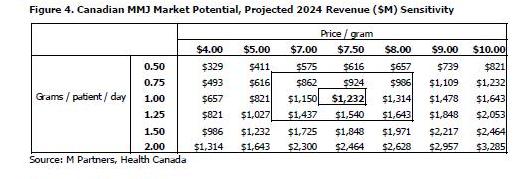
We have projected the total revenue of the MMJ industry in Canada in 10 years to be over $1.2 billion at estimates of 1.0 gram per patient per day and a price of $7.50 per gram. Figure 4 show sensitivities to our calculation that a 0.5 gram per patient per day increase at the same $7.50 price increases 2024 projected revenue by 50%, or $616 million, to a total of $1.8 billion, and that a $0.50 increase in price at the same 1.0 gram per patient per day increases 2024 projected revenue by 6.5%, or $82 million, to a total of $1.3 billion. Health Canada’s projections in the MMPR use a starting price of $7.60 per gram that increases over the next ten years (we’ve assumed a constant average price of $7.50).

RISKS AND OBJECTIONS
The MMJ market is relatively new and largely unproven. The adoption rate of commercial MMJ by qualified patients is difficult to determine but a portion (approximately 13%) of the qualified patient population is already conditioned to using the government contracted service under the old system. Furthermore, the convenience of a wide selection of MMJ strains delivered directly to patients in a discrete and concealed package should be attractive. Healthcare practitioners are key stakeholders as they will be signing and providing the medical documentation needed for patients to register with commercial producers. Regulation under the MMPR should not be vastly different for healthcare practitioners already familiar with the process under the former MMAR. Licensed producers should be held responsible for quality of the product provided as the MMPR outlines strict rules for quality assessment and control, cleanliness, manufacturing, and pesticide use. Security and diversion to the black market remain a concern but MMPR outlines strict rules for segregation of duties and security clearances, background checks for employees and officers, tracking of product in and out of the premises, and camera surveillance.
On a political level, federal court judge Michael Manson granted on March 21, 2014 an injunction from a group of MMJ patients who asked to preserve the status quo until a constitutional challenge of the new system can be heard. The patients’ counsel argued that the new system is forcing them to choose between liberty, which they could lose if they continue to grow their own, and their health, which could deteriorate if they are not able to afford their regular doses under the new system. A change in the majority government could also influence the direction of the program if the Liberals were to take control over the Conservatives.
Though the risk that the MMPR is repealed from this injunction still remains, Health Canada continues to issue licenses to commercial producers. Under a scenario where the old system, MMAR, is back in effect and where individuals with PUPLs and DPPLs are allowed to personally grow, we envision that licensed producers can be still be successful. A minority of patients used the government contracted service under the old system but complained of issues raised earlier in this report, such as a small number of MMJ strains to choose from and a burdensome application process. Licensed producers under either scenario would still be heavily regulated by Health Canada. Licensed producers should still be able to capture a portion of the current PUPL and DPPL segment based on their ability to offer patients diverse types of product at competitive prices with a discrete right-to-your-door service that prevents patients from going through the effort of growing themselves or purchasing through illicit sources.

LOOKING FORWARD
The race for licenses is on. Producers with commercial potential submitted applications to Health Canada months ago and now companies big and small are switching strategic focus to one day be home to a MMJ grow-op. Licensed producers are racing to capture patients while we believe that only four of the thirteen are actually shipping. Health Canada has intended that MMJ become more like a medication by creating a licensing scheme; as marijuana becomes more of a commodity, opportunities to capture incremental profit will arise. Our market estimate above did not consider expansion of the industry with other forms of MMJ including but not limited to edibles, consumables, creams, oils, extracts and derivatives that we have not accounted for. We have also not considered the impact of a decriminalization, or even legalization, of marijuana – a situation in which successful licensed producers would have a much further advantage having already established (expected) commercial sales.
SUMMARY
The regulatory reform of the Marijuana For Medical Purposes Regulations (MMPR) began on April 1, 2014, and all authorizations and licenses issued under the MMAR are no longer valid, but the injunction remains an overhang. The number of qualified patients in Canada is expected to grow ten fold in the next ten years and only licensed producers are allowed to distribute to this patient population. Health Canada has issued 14 licenses so far and could be issuing more soon.
The X-factor for companies thus far licensed by Health Canada is economics – the companies chosen must be capable to grow, sell, and quickly scale the business. Recall a motivation of implementing MMPR is to consolidate the supply chain and reduce the administrative burden of running the program as the population of qualified patients grows. Even though there is no limit to the number of licenses that will be granted, Health Canada is more likely to desire a fewer number of larger capacity producers opposed to many small producers, so first movers have a strong advantage. Investors would be wise to identify legitimate licensed businesses when looking to play this space.

[disclosure]
Comment
Leave a Reply
You must be logged in to post a comment.


 Share
Share Tweet
Tweet Share
Share




There are 22 licenses actually issued. Some are not listed on HC.
Thank Heavens the Underground economy is alive and well…
Honestly people will still sell it illegally..
Do you know if Cen bio Tech is one, stock symbol fitx. They are in lakeshore.
No they are not one
As a result of ongoing litigation and uncertainty arising from court decisions, Health Canada will treat the following Authorizations to Possess, Personal-Use Production Licences, and Designated-Person Production Licences as extending beyond March 31, 2014 until a decision in Allard is rendered. As per the Federal Court interim injunction, the following criteria must be met:
Individuals must have held a valid Authorizations to Possess under the MMAR on March 21, 2014.
Individuals must have held a valid Personal-Use Production Licence or Designated-Person Production Licence under the MMAR on, or after, September 30, 2013, where there is also an associated valid ATP as of March 21, 2014.
Individuals with a medical need who do not fall within the scope of this court order and who have the support of a licensed healthcare practitioner may register with a licensed producer under the MMPR. See below for information on how to access marijuana for medical purposes from a licensed producer.
How do you know there are 22 issues lmao!! The rest of the world is in the dark…you must be the one at HC issuing the licenses lol ..dumb ass!!
Because I speak with officials at health Canada