Medicenna Therapeutics has “elegant science”, Research Capital says
New clinical data from Medicenna Therapeutics (Medicenna Therapeutics Stock Quote, Chart, News, Analysts, Financials TSXV:MDNA) is strengthening confidence in its lead cancer therapy, positioning the company as a notable player in the next wave of immuno-oncology treatments.
On April 28, the company received a Speculative Buy rating and a C$3.75 12-month price target from Research Capital analyst Andre Uddin following the release of new positive interim data from its MDNA11 clinical trial.
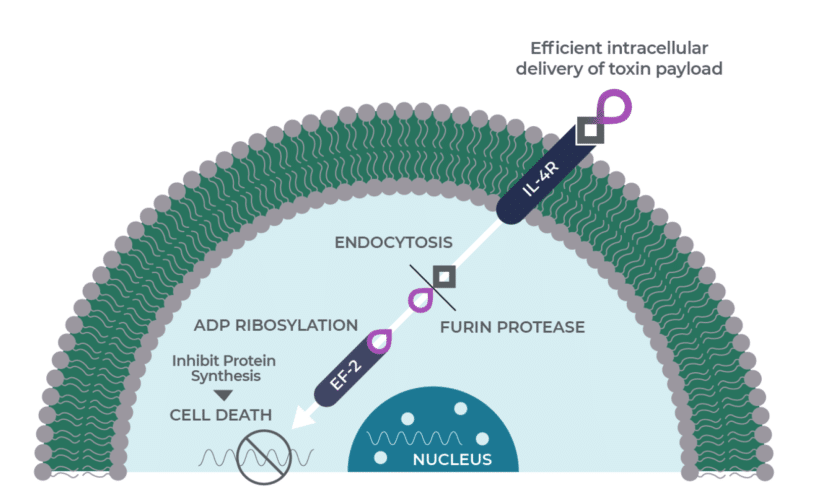
Medicenna presented updated Phase 1/2 results for MDNA11 at the American Association for Cancer Research (AACR) Annual Meeting on April 27. The company said the combination of MDNA11 and Keytruda achieved a notable objective response rate (ORR) of 36% across all tumour types, up from 22% reported in December 2024, while maintaining a favourable safety profile with more than 90% of treatment-related adverse events being mild or moderate.
“We are encouraged by the anti-tumour activity and safety of MDNA11 in both the monotherapy and combination setting, including the three recent responders, particularly in patients who have shown resistance to checkpoint inhibitors or with tumour types with historically low responses to immunotherapies and addressing a vastly underserved cancer population,” Medicenna president and CEO Fahar Merchant said in a press release discussing the results released at the AACR agm. “Today’s efficacy and immunodynamic data have provided the foundation to commence the dose expansion portion for MDNA11 in combination with KEYTRUDA.
“In the monotherapy setting, we continue to see deep and durable single-agent activity of MDNA11 with two patients remaining in complete remission and off therapy. Together, these data reinforce the best-in-class potential for MDNA11 as a potent and safe immunotherapy that is poised to transform the existing treatment paradigm for patients with difficult-to-treat tumours.
“We look forward to sharing additional clinical data from the ABILITY-1 study at future medical conferences this year.”
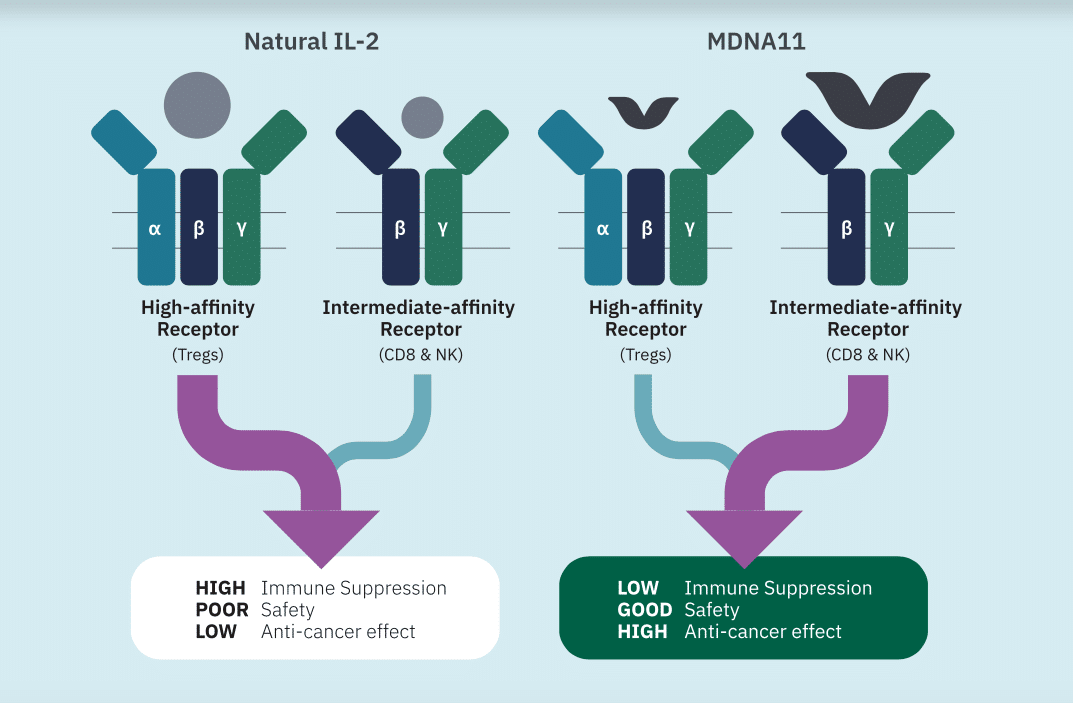
Uddin said the data strengthens the case for MDNA11 as a leading next-generation IL-2 therapy in solid tumours.
“MDNA11’s safety profile, coupled with its strong interim efficacy, differentiates itself from other IL-2 drug candidates that generated weak efficacy data and poor safety profiles,” Uddin wrote.
Uddin said that Medicenna won’t generate any revenue in fiscal 2025 or 2026, as the company is focused on clinical development and does not anticipate material product revenue yet.
The analyst added that assuming MDNA11 continues to deliver strong data, a licensing deal could happen as early as 2027, potentially transforming the company’s valuation outlook.
“We remain bullish on MDNA’s elegant science and expect further data throughout calendar 2025,” Uddin said. “Assuming that MDNA11 continues to generate compelling clinical data, we would anticipate a licensing deal by early 2027.”
-30-

Rod Weatherbie
Writer
Rod Weatherbie is a journalist based in Prince Edward Island. Since 2004, he has written extensively about the Canadian property and casualty insurance landscape. He was also a founder and contributing editor for a Toronto-based arts website and a PEI-based food magazine. His fiction and poetry have been featured in The Fiddlehead, The Antigonish Review, and Juniper.