

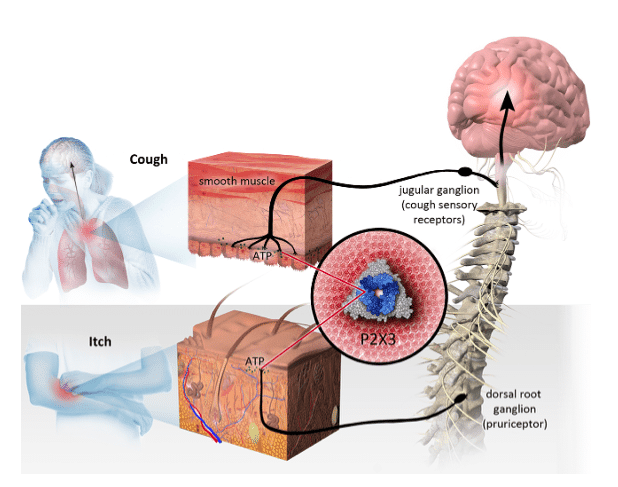
On Monday, Bellus reported results from a Phase 1 study of its BLU05937 drug for the treatment of chronic cough, finding that the drug has a good safety and tolerability profile and a pharmacokinetic profile that supports twice-a-day dosing.
“The clinical data confirm our expectation that at the anticipated therapeutic doses there is no or very limited effect on taste perception and further reinforce our position that BLU-5937 has the potential to be a best-in-class therapeutic for chronic cough patients,” said Dr. Denis Garceau, Senior Vice President of Drug Development for Bellus, in a press release. “We believe the Phase 1 data provide strong support for our planned clinical Phase 2 study for BLU-5937, which we expect to initiate in mid-2019.”
Uddin says the results put Bellus in the position to potentially license BLU-5937 or to itself be an acquisition target.
“In June 2016, Merck acquired Afferent for US$1.25 billion to obtain MK-7264 (formerly known as AF-219), gefapixant,” says Uddin. “These strong Phase I safety results indicate that BLU-5937 has much higher selectivity toward P2X3 than MK-7264 which should help Bellus forge a licensing deal earlier than we expect (the company could also be acquired). To be conservative, we have assumed Bellus would out-license BLU-5937 in 2020 – post Phase II.”
Uddin notes that a 2015 study found 11 per cent of adults in the United States have chronic cough, with five per cent of chronic cough cases defined as having an unknown cause, resulting in a 1.5-million addressable patient population in the US. The analyst estimates a launch date of 2023 for BLU-5937, with global sales estimates of US$41.6 million in 2023, US$147.8 million in 2024 and US$266.9 million in 2025.
Leave a Reply
You must be logged in to post a comment.




 Share
Share Tweet
Tweet Share
Share




Comment