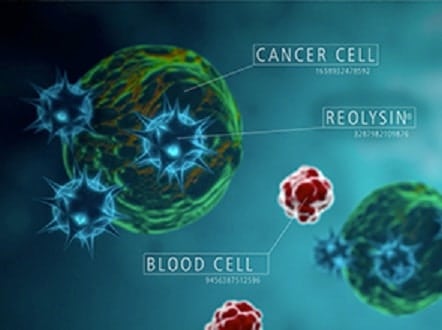
Yesterday, after market close, Oncolytics (TSX:ONC, Nasdaq:ONCY) delivered underwhelming data from a Phase II cancer study, causing its stock to swoon today.
The U.S. National Cancer Institute sponsored study evaluated Oncolytics experimental therapy, REOLYSIN, in combination with paclitaxel versus a control arm that included carboplatin and paclitaxel in patients with recurrent or metastatic pancreatic cancer. The study results showed that progression free survival, the length of time during or after medication that a patient’s cancer does not get any worse, was virtually identical between the REOLYSIN arm and the control arm; 5.26 months for REOLYSIN arm and 5.16 months for the control arm.
Oncolytics did present additional data for a subgroup of patients with a certain gene mutation known as KRAS. In this patient population, the REOLYSIN arm showed a small benefit in progression free survival versus the control arm; 5.72 months versus 4.11 months.
The pancreas is only one of many organs where Oncolytics is testing the potential anti-cancer benefits of REOLYSIN. On their conference call the company’s executives expressed enthusiasm about upcoming REOLYSIN data read outs for ongoing colorectal, ovarian and lung cancer studies. However, for today at least, investors are focused on Oncolytic’s pancreatic data, and its implications on other REOLYSIN studies.
At press time, shares of Oncolytics Biotech on the TSX were down 39.3% to $0.85.
Leave a Reply
You must be logged in to post a comment.





 Share
Share Tweet
Tweet Share
Share




Comment