 Negative top-line results from its Phase 2 clinical study are cause for a rating change on
Negative top-line results from its Phase 2 clinical study are cause for a rating change on
Bellus Health (Bellus Health Stock Quote, Chart, News TSX:BLU), says Mackie Research analyst André Uddin, who on Monday downgraded Bellus from “Speculative Buy” to “Hold.”
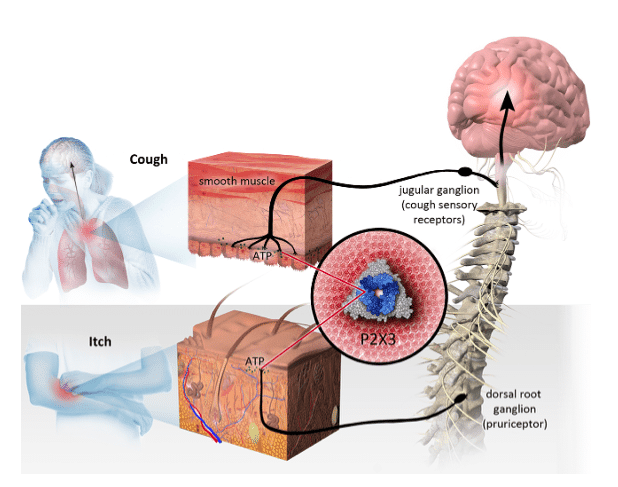
Shares of Laval, Quebec’s Bellus Health dropped like a stone on Monday with the release of results from its Phase 2 RELIEF trial of BLU-5937, a P2X3 receptor antagonist and the company’s chronic cough drug candidate which is targeting a multi-billion dollar market opportunity.
As Bellus reported, BLU-5937 missed the primary endpoint of the study, awake cough frequency, and was testing four doses of the drug in refractory chronic cough patients at 25 mg, 50 mg, 100 mg and 200 mg. Bellus said BLU-5937 did not achieve statistical significance for the primary endpoint of reduction in placebo-adjusted cough frequency at any dose tested.
Bellus reported a subgroup analysis which showed the drug’s efficacy was positively associated with patients’ baseline cough frequency, with Bellus saying the candidate achieved highly significant cough reductions at all doses in subjects with a baseline cough frequency of over 32 coughs per hour.
“In the RELIEF trial, we observed data that we believe is competitive within the P2X3 class, including the reduction in cough frequency shown in patients with higher cough counts and a low taste effect. While we had hoped to see more response in the lower cough patients, BLU-5937 and other P2X3 antagonists may have the most benefit in patients with a greater disease burden,” said Roberto Bellini, President and CEO of Bellus, in a press release.
“We believe the Phase 2 data support moving BLU-5937 forward into an adaptive Phase 2b trial enriched for higher cough count patients. We expect to begin this trial in the fourth quarter of 2020,” Bellini said.
In his assessment, Uddin said the study leaves some key questions to be answered.
“P2X3 should be a validated target in chronic cough given positive efficacy results were achieved by rival P2X3 inhibitors (Merck and Shinogi). Two key questions: (i) What went wrong in RELIEF? – a theory is that the trial included too many patients with low baseline cough frequency, which would dilute the efficacy of BLU-5937, and (ii) Why was there no evident dose-dependent response in awake cough frequency? – a theory is that BLU-5937 may have already approached P2X3 occupancy plateau at 25mg,” Uddin wrote.
Uddin mentioned that Bellus is looking to discuss the RELIEF results with the US FDA sometime during the fourth quarter, along with the launch of a Phase 2b trial for BLU-
5937 in the same quarter.
The analyst’s new “Hold” rating came with a reduced target of $8.70 per share (previously $16.30). The stock closed Monday down 72.3 per cent to $4.53.
“We are pushing out our assumed timeline and financial estimates for BLU-5937 as the market opportunity may not be as large as what we had forecast,” Uddin said.
Leave a Reply
You must be logged in to post a comment.






 Share
Share Tweet
Tweet Share
Share




Comment