

ProMetic Life Sciences’ (ProMetic Life Sciences Stock Quote, Chart, News: TSX:PLI) IPF treatment could be a company maker, says Canaccord Genuity analyst Neil Maruoka.
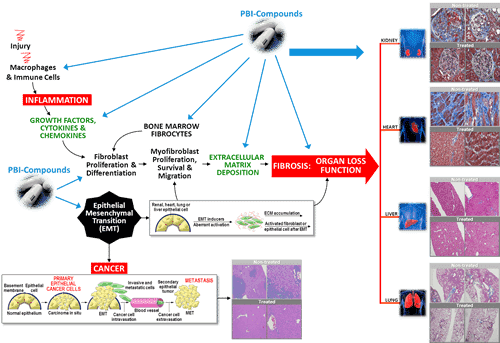
On Monday, ProMetic announced that its oral anti-fibrotic lead drug candidate, PBI-4050, had received FDAi nvestigational new drug (IND) approval to commence its pivotal phase 2/3 clinical trial in patients suffering from idiopathic pulmonary fibrosis (IPF).
“We are very encouraged by the results of our open-label trial of PBI-4050 in combination with nintedanib, and are pleased to advance the clinical development plan with this pivotal study,” said chief medical officer. Dr. John Moran. “IPF is a very serious condition and we believe that patients may benefit from this novel therapeutic approach. We have multiple key opinion leaders who have expressed a wish to participate in the study, and now that the IND has been cleared we can begin a formal study start-up.”
Maruoka give thumbs up to this development.
“We view this as incrementally positive, as the IND approval from the FDA provides modest validation for this program, the analyst says. “Moreover, we believe the adaptive study design could represent an abbreviated path to commercialization in this potential blockbuster indication. The study will evaluate ‘4050 together with Boehringer Ingelheim’s nintedanib, a combination that produced encouraging results in a small open-label Phase II study last year. While we expect that ProMetic will be able to initiate the Phase II portion of the trial on its own, given the large size of the study and ProMetic’s elevated cash burn we expect the company will need to establish a partnership to complete this study. Although timelines have slipped, we continue to see a number of catalysts lined up for ProMetic in coming months including more data from PBI-4050, the initiation of clinical studies for PBI-4050 in IPF and DKD and, ultimately, the expected FDA approval of Ryplazim.”
In a research update to clients Monday, Maruoka maintained his “Buy” rating and one-year price target of $4.25 on ProMetic Life Sciences, implying a return of 172.4 per cent at the time of publication.
Leave a Reply
You must be logged in to post a comment.




 Share
Share Tweet
Tweet Share
Share




Comment