

New data from ProMetic Life Sciences’ (ProMetic Life Sciences Stock Quote, Chart, News: TSX:PLI) ongoing phase 2 open-label clinical trial is giving Canaccord Genuity analyst Neil Maruoka more confidence in his bullish target on the stock.
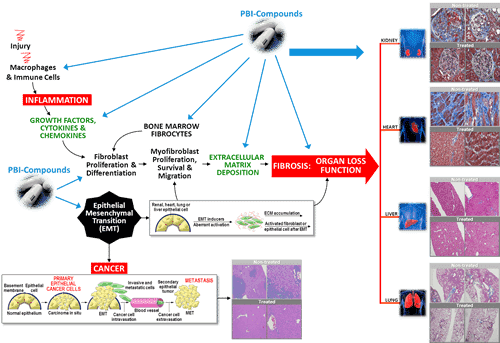
This morning, ProMetic reported what it described as positive clinical data from its continuing phase two trial in which patients suffering from Alstrom syndrome were administered its PBI-4050.
“We have previously reported the encouraging results in the first five subjects who had reached 24 weeks of treatment. We now have results on eight subjects who have had 36 weeks or more of treatment, up to 60 weeks,” said ProMetic’s chief medical officer, Dr. John Moran. “The safety and tolerability has been confirmed over this extended period with no drug-related serious adverse events. The best-accepted non-invasive technique for assessment of liver fibrosis is the FibroScan. In these eight subjects there was a significant improvement in this measure of liver stiffness, from a median of 8.5 at baseline to 5.8 at last measurement, an absolute decrease of 2.7 kilopascals (31 per cent), p equals 0.0098. Accompanying this, we saw a normalization of liver enzymes in those subjects whose levels were elevated at baseline. Furthermore, we observed a major reduction of key biomarkers in the urine of the nine subjects in whom the 24-week results are available. Elevated levels of these biomarkers indicate ongoing kidney injury.”
Maruoka says that even though ProMetic’s timelines have been pushed back he believes these results could help hurry it up once again.
“We believe this data from a total of eight patients supports the potential safety and efficacy of ‘4050 in this indication,” the analyst says. “Recall that ProMetic had previously announced positive data from the first five patients in this study, which showed a significant reduction in liver fibrosis, as measured by Fibroscan. The new data reflects an extended treatment period of 36 weeks (and up to 60 weeks), underscoring the durability of effect in this very difficult patient population. Based on this efficacy data and the lack of treatment options for Alström patients, ProMetic intends to pursue clarity on the steps to approval from the FDA and EMEA. Given the ultra-rare and serious nature of this disease, we believe the regulatory pathway could be shorter in Alström syndrome, with data from this Phase II study potentially supporting approval in this indication.”
In a research update to clients today, Maruoka maintained his “Buy” rating and one-year price target of $4.25 on ProMetic Life Sciences, implying a return of 197.2 per cent at the time of publication.
Maruoka thinks ProMetic will generate and EBITDA loss of $93.9-million on revenue of $32.5-million in fiscal 2017. He expects those numbers will improve to EBITDA of positive $16.0-milllion on a topline of $150.4-million the following year.
Leave a Reply
You must be logged in to post a comment.




 Share
Share Tweet
Tweet Share
Share




Comment