
 A warrant exercise reduces the financial risk of ProMetic Life Sciences (TSX:PLI), says Echelon Wealth Management analyst Doug Loe.
A warrant exercise reduces the financial risk of ProMetic Life Sciences (TSX:PLI), says Echelon Wealth Management analyst Doug Loe.
This morning, ProMetic announced that California Capital Equity LLC had exercised 44,791,488 share purchase warrants at a price of 47 cents per share for total proceeds of $21,051,999.36 to ProMetic.
“Our increased equity position in ProMetic represents a long-term and strategic investment for us,” said Dr. Patrick Soon-Shiong, CEO of California Capital Equity LLC. “ProMetic has robust proprietary therapeutic platforms, promising growth potential and several plasma derived therapeutics expected to reach commercialization stages, starting in 2017 with plasminogen. We, through other investment vehicles, also have an ongoing partnership with ProMetic in relation to IVIG and anticipate further collaborations going forward.”
Loe says this development indicates a substantial reduction in the financial risk around ProMetic Life Sciences.
“Near-term, the proceeds comes at a time when ProMetic is expected to begin ramping up its commercial activities associated with its plasma protein programs,” notes the analyst. “Specifically, PLI’s plasminogen program in patients with congenital plasminogen deficiency could be part of a final BLA filing expected in FH117, in line for formal FDA review in FH217. Further ahead, we expect the IVIG program to complete Phase III trials by H217, in line for a BLA filing submission by FQ417-FQ118.”
In a research update to clients today, Loe maintained his “Buy” rating and one-year price target of $4.00 on ProMetic Life Sciences, implying a return of 90 per cent at the time of publication.
Loe thinks ProMetic will generate negative EBITDA of $63.6-million on revenue of $38.1-million in fiscal 2017. He expects the company will generate negative EBITDA of $66.5-million on a topline of $48.5-million the following year.
Comment
One thought on “ProMetic Life Sciences just got less risky, says Echelon Wealth”
Leave a Reply
You must be logged in to post a comment.





 Share
Share Tweet
Tweet Share
Share




LAVAL, QC, Feb. 22, 2017
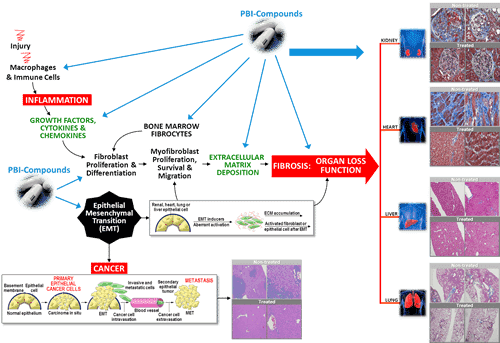
Early evidence of efficacy of PBI-4050 as a monotherapy and in combination with one of the commercially available drugs
confirmed
PBI-4050 continues to be very well tolerated, whether used alone or in combination with nintedanib or pirfenidone
LAVAL, QC, Feb. 22, 2017 /CNW Telbec/ – ProMetic Life
Sciences Inc. (TSX: PLI) (OTCQX: PFSCF) (“ProMetic” or the “Corporation”) announced today positive results from
its completed open label Phase 2 clinical trial in subjects suffering from idiopathic pulmonary fibrosis (“IPF”).
In
addition to demonstrating that PBI-4050 is safe and very well
tolerated, an objective of this study was to seek early evidence of
a clinical benefit with PBI-4050 treatment, whether administered alone
or in addition to either of the drugs approved for the
treatment of IPF, nintedanib or pirfenidone. These results confirm the
preliminary results previously announced by Prometic on
November 17, 2016, following the first 30 subjects’ completion of 12 weeks of treatment.
A total of 40 subjects were enrolled in the study conducted in 6
sites across Canada and all have completed the 12 weeks of
treatment; 9 subjects received PBI-4050 alone, 16 received PBI-4050
& nintedanib and 15 received PBI-4050 & pirfenidone.
The baseline characteristics of the subjects enrolled in this study
were similar to those enrolled in prior IPF randomized
controlled studies conducted by other pharmaceutical companies, namely
ASCEND and INPULSIS.
As was demonstrated in these previously mentioned large clinical
trials, IPF subjects typically experience a progressive
decline in respiratory function. In contrast, in the ProMetic clinical
study, the respiratory function of the subjects, measured
as the forced vital capacity (FVC (ml)), remained stable after 12
weeks of treatment, in subjects treated with PBI-4050 alone and
in those receiving PBI-4050 combined with one of the two approved
drugs for the treatment of IPF (“Combi-1”) and was superior to
that of those subjects treated with PBI-4050 combined with the other
approved drug for the treatment of IPF
(“Combi-2”).
“PBI-4050, either used alone or in Combi-1, demonstrated very promising early indications of efficacy, considering that
current drugs approved for IPF only slow (but do not reverse) the decline in lung respiratory function”, commented Dr.
John Moran, Chief Medical Officer of
ProMetic. “It is also important to note that during our
clinical trial, there were no deaths nor did we see any subjects
experiencing a decrease in FVC of 10% or more, contrary to the
outcomes in the other IPF trials. There were no serious adverse events
requiring PBI-4050’s discontinuation. The most frequent
adverse event seen in all groups was diarrhea, but this was clearly
much less significant in the subjects treated with PBI-4050
alone than in the groups receiving either of the currently approved
drugs for the treatment of IPF, which are well-known for
their significant side effect profiles”, added Dr. Moran.
Pierre Laurin, ProMetic’s President and Chief Executive Officer commented: “These positive
results support the rationale and clinical study design for the placebo controlled, pivotal Phase 2/3 IPF clinical trial we
intend to initiate in Q2 2017. We expect to see PBI-4050, alone or in combination with one of the commercially approved IPF
drugs, continue to outperform the current drugs in terms of efficacy, safety and tolerability”.
The comparisons shown herein between the results in this study and other larger phase 3 clinical studies are only made to
provide some provisional guidance in terms of the potential clinical benefits of PBI-4050 for IPF patients.
Please note that the complete data set for ProMetic’s open label Phase 2 clinical trial will be presented at an upcoming
pertinent scientific conference, notice of which will be given by the Corporation.