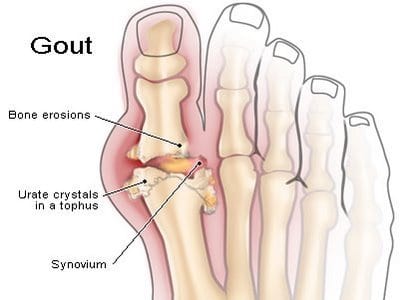
 Revive Therapeutics (TSXV:RVV) today announced that it has requested a pre-investigational New Drug meeting with the FDA for its gout drug candidate, REV-002.
Revive Therapeutics (TSXV:RVV) today announced that it has requested a pre-investigational New Drug meeting with the FDA for its gout drug candidate, REV-002.
The Ontario-based drug repurposing junior says the request follows on its material transfer agreement with an unnamed Japanese global pharmaceutical company that allowed Revive to obtain access to confidential information and clinical trial supply for a US-based trial.
“This request marks another important step in Revive’s program to obtain US FDA approval for REV-002 in the treatment of gout,” said CEO Fabio Chianelli.
Gout is a condition that causes elevated serum uric acid in the body due to under excretion of uric acid and/or over production of uric acid. There were 14.3 million diagnosed prevalent cases of chronic gout in the major pharmaceutical markets in 2012, a number that is forecast to rise to 17.7 million by 2021.
Revive, which was founded two years ago and listed on the TSX Venture Exchange earlier this year, is also currently developing and commercializing treatments for afflictions such as sleep apnea and rare diseases.
At press time, shares of Revive Therapeutics were up 20% to $0.60.
Disclosure: Revive Therapeutics is an annual sponsor of Cantech Letter.
Leave a Reply
You must be logged in to post a comment.





 Share
Share Tweet
Tweet Share
Share




Comment