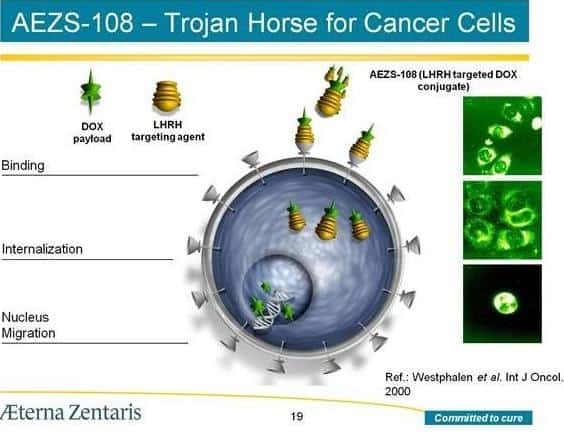
After the disappointment of its lead offering, perifosine, earlier this year, shares of AETerna Zentaris are showing some life today after the company announced it has reached a key agreement on another treatment in it stable; AEZS-108.2012 was a year to forget for AEterna Zentaris (TSX:AEZ).
Things were on track until April, when the Quebec-based oncology/endocrinology drug maker announced that its lead offering, perifosine, failed to meet its primary endpoint.
On May 7th, partner Keryx returned the rights to the treatment to AEterna Zentaris. Shares of the company fell from $2.14 on March 30th to lows under $.40 before a six-for-one share consolidation early in October.
Today, shares of AETerna Zentaris are showing some life again, after the company announced it has reached a key agreement on another treatment in it stable; AEZS-108.
The United States Food and Drug Administration, on a special protocol assessment, has approved a phase 3 registration trial in endometrial cancer with AEZS-108.
CEO Jurgen Engel said the trial was a fresh start for the company.
“We are pleased with the agreement with the FDA which provides us with a clearly defined development and regulatory pathway for AEZS-108 in endometrial cancer,” he said. “AEZS-108’s innovative targeted approach could offer a new treatment option for women with endometrial cancer and provide the company with a significant market opportunity.”
AEterna Zentaris says the trial will be an open-label, randomized, multicentre phase 3 trial conducted in North America and Europe. Approximately 500 patients will be involved.
At press time, shares of AEterna Zentaris on the TSX were up 15.7% to $2.50.
_______________
Leave a Reply
You must be logged in to post a comment.






 Share
Share Tweet
Tweet Share
Share




Comment