
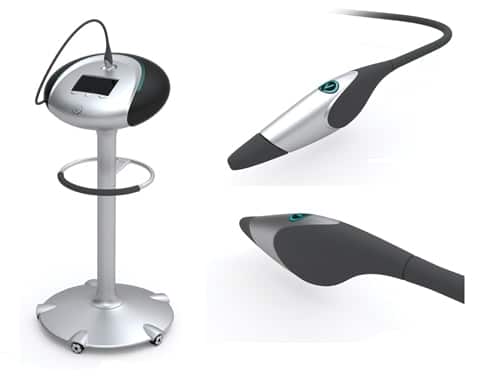
For Vancouver’s Verisante (TSXV:VRS), the waiting game is almost over. The company’s skin cancer detection device, Aura, seems on the cusp of moving from concept to practice, as important regulatory approvals are expected to pass later this year.
Rainy Vancouver might seem an odd point of origin for science that may be set to turn the multi-billion dollar skin cancer market on its sunburned ear. But this fact seems much less curious when one notes the company’s deep ties to the UBC Department of Dermatology and Skin Science, which is a world leader.
On a partly-cloudy day at Verisante’s Vancouver head-office, Cantech Letter’s Nick Waddell sat down with Verisante CEO Thomas Braun.
Thomas, A lot has changed recently for Verisante. You are more than a year past your deal with the BC Cancer Agency, and you are now on the cusp of commercialization. Can you bring us up to speed on what’s been happening?
We actually reorganized the company, so we could devote more resources to the engineering and the development of the product, as opposed to people who worked in the office, administrative staff. We downsized that side of the company quite severely, reducing those costs by about 50%. We then allocated those resources to engineering. We hired a team, led by Branko Palcic, who was the founder of the imaging lab at the BC Cancer Agency, and also founded the technology development office, and all the people who work there now were hired and mentored by him. The main inventor of the device, on the physics and engineering side, Dr. Haishan Zeng, became our project leader. We then entered into a bunch of collaborative research agreements with the BC Cancer agency and UBC. We got a matching grant from the Canadian Institute of Health Research, and that money has been used to develop the probe so it can be commercialized. We made a number of improvements and needed to complete a statistical analysis and bring the data up to date. That was finished in May, but we haven`t released it yet because we are waiting for UBC to publish it.
So where is the Aura device at now, has it changed throughout the process of proving out the science?
When we came into the picture, we just had the clinical prototype that was built seven years ago. All the parts were state of the art seven years ago, but there has been a lot of improvement in digital cameras, computers are way better now, everything is faster. We had to put together the alpha prototype and figure out which components we could make ourselves and what we should buy off the shelf and who we should buy it from. We went through the process of testing different things and trying different things out. We basically put together a core group of engineers at another office in Richmond, BC, where we now have about half a dozen people. These are guys who have experience building devices for companies. When, for instance, the BC Cancer Agency needs something built, they would go to this engineering firm. They have built things for Perceptronix, Xillix, and for some very large companies in China.
Fortunate turn of luck for you that the group is here..
Yeah, we took over that small engineering firm and they now work for us. The space they were renting, we have now leased. They have gone through a bit of a trial and error process to build the prototype and set the specs for the device. The next step is to make an even more refined version, which we call the beta prototype and that beta prototype will be the final specifications that we will go to market with. We also use these prototypes to do all the safety testing. So the clinical prototype that is used for the clinical study has actually never been through all the rigorous safety testing that is required for products that are sold commercially. That involved taking these prototypes and sending them to engineering firms that are certified to do this kind of work. They have a fifty page checklist with about five-hundred different boxes of things they have to check off as they test them. So all of that has been done over the last few months. It’s required by Health Canada and for a CE Mark.
What about on the financing front, you have been pretty active there.
Yep, at the beginning of this year we raised a million dollars and then $5 million in April. On top of that we made money from the warrants that were in those offerings, to date $2.7 million worth of those warrants have been exercised. The February offering was $.25 cents a unit and the warrants had an exercise price of $.30 cents and those have all been exercised. Now it’s just the $.50 cent warrants from April that are out there. We’ve had five million warrants exercised, which is nice. We have been getting a few hundred thousand dollars every month since April. Ever since we did that financing we haven’t had to touch our original capital, the warrants have covered our burn rate. We have raised a total of $8.7 million, which gives us a strong financial position.
So, you’re entering a new stage of the company’s history…
Yes. We’ve got the alpha prototype done, we’ve got the collaborative statistical analysis with UBC that has resulted in an article that has been drafted by the head of the UBC Dermatology Department, Dr. Harvey Lui, who is the principal investigator. As I said, we’re just waiting to publish that. Meanwhile we got our ISO certification, which was announced in late July. The nice thing about that is that for the CE marking for Europe and for Health Canada approval you are required to have this ISO certification. If we didn’t have that ISO certification we would have to rely on another company to apply for regulatory approval on our behalf, because the applicant has to have that certificate, even if you are using an OEM who is going to do the manufacturing for you, which is our plan at this point. We made a decision to use an outside manufacturer so we can keep our costs as low as possible. You just don’ t know long it is going to take for demand to ramp up. Every company hopes for the best and you’ve got your projections, but you don`t want to risk having huge overhead, a warehouse full of devices without the orders to match it. We’re using an OEM in Victoria called Starfish. They’re a long established firm who have just bought their own building. They figure they can manufacture as many as forty units a month for us.
So you will really never have a ton of inventory…
Nope, we will do small production runs. What I have seen from some peers such as Novadaq, who do some of their manufacturing themselves, installing electronics, is that they have a very high burn rate. Even though they are now selling and doing really well they’re still losing money. If they didn’t have that huge overhead they would be a profitable company and their stock would be even higher than it is, I suspect.
It seems as though you have put a lot of thought towards this transition to commercialization. When did you start thinking about these things?
We started thinking about it right after we signed the agreement to commercialize the device, in July 2010. I knew then that the kind of work that needed to be done was industrial engineering, the scientific work was basically completed. At that time we didn’t really have a revenue model worked out either. Investors were saying “We like your technology, but what is your regulatory pathway, your commercialization pathway?” In fact, the key to unlocking our business model is the approvals. The CE Mark gets you into 27 countries in Europe, and Australia. And Health Canada approval is very important. It gets you into Canada but many other countries will accept Health Canada approval without requiring any additional clinical studies. For example, Brazil will accept Health Canada approval.
You`re at the stage right now where its important to allocate your resources properly and enter the correct market first. You have told me about the importance of the Australian market in the past. Is this where sales of Aura will begin?
Australia is the skin cancer capital of the world. The country is interesting and it is different from the rest of the world. In the United States and the UK dermatologists have a stranglehold on treating skin cancer. In Australia there is something like just 250 dermatologists for the entire country. There is a severe shortage. Some people would say the reason is that the dermatologists have set up a closed shop. The dermatologists themselves have to train more, so its a bit of a union situation. The training spots for dermatologists are funded by the federal government. Contrary to what some might think, the US government does this as well. In Australia it takes seven years after medical school to become a dermatologist. So there is a shortage and a very difficult path to fix the shortage.
So what does this difficult environment mean for you?
What is means is that there is great demand for a device that GP’s can use. In Australia they have something called the Australasian College of Skin Cancer Medicine. This is basically a skin cancer medicine college that competes with dermatology. These are people who are technically GP’s, but they have taken extra training from the skin cancer college, they’ve written exams and they’ve been certified in skin Cancer medicine. They can also take additional courses and exams to achieve diploma status, and they can keep on going and become Fellows. To get a fellowship is very hard, I think there are only four or five in the whole country. So you have a two-tired system in which you have GP’s who are basically skin cancer specialists, they do nothing but treat skin cancer all day long. They’re not dermatologists, but they have skin cancer clinic and they see sixty patients a day. They do screening, biopsies and the treatment. They do radical surgery that in Canada or the US only a plastic surgeon would be allowed to do. A patient might have to have half their ear or part of their nose cut out and they do this reconstructive plastic surgery. So it`s a little bit misleading to call them a GP, it underestimates their ability. So while there are a little more than two-hundred dermatologists in Australia, there are over a thousand of these GP’s who specialize in skin cancer medicine.
Verisante CEO Thomas Braun interviewed by Tracy Byrnes of Fox News:
So a very evolved, mature market…
Yeah, there is just a ton of awareness of skin cancer. Just about every other person you meet there, it seems, has had just had something removed. A very, very high percentage of Australians get some form of skin cancer in their lifetime. Another thing that benefits us in introducing our device into this market, is that the cities in Australia represent a very large percentage of the overall population. This concentration is very nice in that we won’t have to have our sales force spread around too much. I think that if we can find the right distributor or set up our own marketing network, we can start with just Sydney, Brisbane, Melbourne and Perth.
So when do you enter the Australian market?
Australia has a reciprocal agreement with Europe where they recognize the CE Mark and the process requires us to register our device once we have that CE Mark. That can take a couple months, unless they decide to audit you. Once in a while they will pluck device out start to ask questions. And that might happen to us because this is not a run of the mill product, this is a game-changing technology that is going to be of huge national importance to Australia. So we may get a closer look and it may take longer than a couple months. But that’s to be expected if you’re doing something as important as what we’re doing. Also, this is cancer. The consequences of having a device that doesn’t work, that people are relying on, is great. This is something that people pay more attention to because it is a game changer. Australia is going to be a good market, and I’m excited about it , but it’s also important for me to get acceptance from the people I think are world’s experts in skin cancer. It’s the guys in Australia who are running these skin cancer clinics, they’re not running a university hospital , they not at The Mayo Clinic or whatever, they are in the trenches. I think that once we get going in Australia it will be hugely popular and we’ll sell a lot of devices. It will take some work, but a key strategy is to get the thought leaders to buy into what we are doing.
Let’s talk about the value proposition of your device. What does Aura cost and how long will it take a doctor to pay it off?
The device will come in around the $60,000 mark, and I have no doubt that doctors will be able to afford it. When you look at competing type devices, doctors are paying $80-$110,000 for a laser that does laser hair removal. They’re paying $90,000 for machines that do non-surgical liposuction. For a one hour treatment on one these machines, here in Vancouver, they`re charging between $1300-$1500. One thing that has become clear is that whatever I am thinking about they will charge, dermatologists are thinking of charging much more. I was just a lawyer, I charged people $300 an hour when I practiced law. To charge someone $1500 to sit on a machine that freezes your fat for an hour, to me is something that I didn’t think someone could get away with. To charge $1000 for a ten minute botox sitting. Normal people have no idea what these people are paying. Doctors using Aura will probably charge $500 for a screening, if its not covered in Canada, for instance, under the Canadian Health Act they can charge whatever they want because there is no billing code for it. And the dermatologists I have talked to here don’t want a billing code, they want to charge whatever they want. And people will pay. When you talk to anyone who has had skin cancer, they will pay just to have piece of mind.
So let’s kind of calendar out what you expect to happen within the next year…
Between now and mid-2012 we’ll be approved in Canada, well be approved in Europe and in Australia and Starfish will be in full production. We expect to have the regulatory approvals before the end of this year, if all goes well. We don’t anticipate having a lot of troubles with these approvals because the device comes from a government agency and was tested for six years. We will start pre-marketing by going to dermatology and medical device conferences and trade shows. A lot of people simply won’t buy it until they can get a live demonstration, we expect that by March we will have fully functioning models from Starfish that we can take to trade shows and demonstrate for delivery in June. We should be generating revenue starting in eight months. Before the end of this year we will have seven fully functioning prototypes that we will send out to leading clinics in Australia, the UK, to Cambridge, and in the United Sates. We’ll use this to collect tons of data and then use that data to try and get FDA approval.
______________________________________
______________________________________
Leave a Reply
You must be logged in to post a comment.






 Share
Share Tweet
Tweet Share
Share




Comment