Shares of Eupraxia (Eupraxia Stock Quote, Charts, News, Analysts, Financials TSX:EPRX) have rallied hard over the past three quarters, but investors can expect even more upside from the clinical stage biotech company, according to Raymond James analyst Rahul Sarugaser. In a Tuesday report, Sarugaser maintained an “Outperform” rating and $11.00 target price, representing at the time of publication a one-year projected return of 125 per cent.
The topic of Sarugaser’s report is the just-announced agreement for pharma giant GSK to acquire Quebec-based Bellus Health for US$2 billion. GSK is banking on the success of Bellus’ camlipixant, a Phase 3 asset for treating refractory chronic cough, a condition with no currently approved medicines in either the US or the EU.
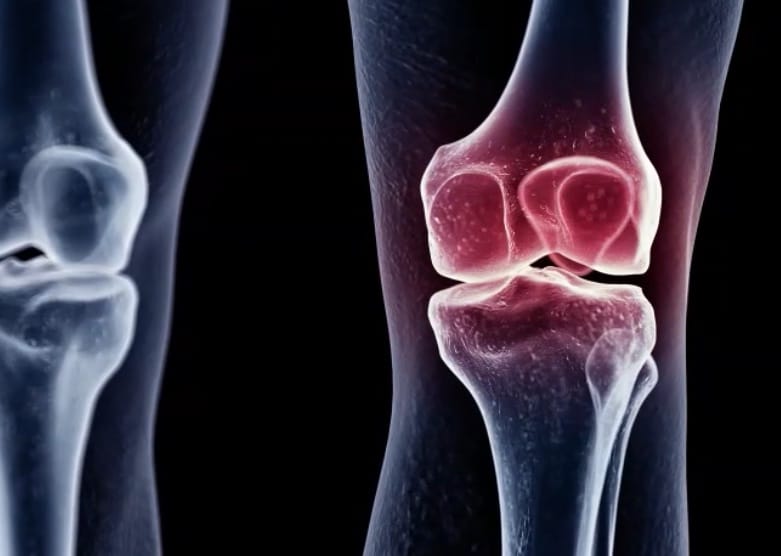
For Sarugaser, the parallels are there between Bellus and Eupraxia. The former has a product which is aimed at addressing an unmet need with a total addressable market of about six million in the US and EU, while the latter has lead clinical candidate EP-104, a drug-polymer combination that slow-releases the steroid fluticasone. Eupraxia currently has EP-104 in a Phase 2 trial for osteoarthritis (OA) of the knee, with a key data readout expected during late second quarter 2023.
Sarugaser said conversations with key opinion leaders suggest the vertical market for steroid injections addressing OA of the knee are at about seven million patients, and the analyst suggests that, like camlipixant which is being pegged as a potential first line therapy, EPRX’s drug “could very well elevate to first line status.”
“How deeply EPRX’s EP-104 will penetrate this market is squarely dependent on the strength of its imminent Phase 2 data read-out, particularly its duration of pain relief. Other factors that will dictate EP-104’s market penetration potential—which will likely be revealed through EPRX’s registration trial—will be its suitability for multi-dosing, and its capacity to spare cartilage,” Sarugaser wrote.
Sarugaser also argued that like camlipixant, whose well-defined mechanism of action likely supported GSK’s confidence in acquiring the drug ahead of Phase 3 readout data, EP-104 involves a well-known corticosteroid with a lot of on-market data to show its anti-inflammatory effects, thus de-risking the asset, according to Sarugaser.
“The key questions remaining: How well/for how long? And, with what safety profile?” Sarugaser said. “From our perspective, EP-104’s proprietary diffusion-driven drug release vehicle and its delivery of a well-understood corticosteroid has a good probability of yielding long-duration pain relief with a limited adverse event profile.”


 Share
Share Tweet
Tweet Share
Share



Comment