
Alpha Cognition (Alpha Cognition Stock Quote, Charts, News, Analysts, Financials TSXV:ACOG) shares are down significantly over the last year and a half, but it may be a while yet before there’s any life in the stock, according to Raymond James analyst Rahul Sarugaser. In a Thursday report, Sarugaser downgraded ACOG from “Outperform” to “Market Perform,” saying a New Drug Application approval may still be a year out.
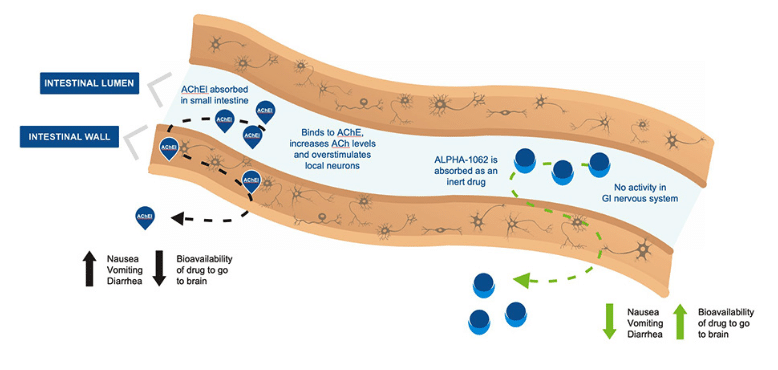
Vancouver-based biopharma company Alpha Cognition is in the late stages of developing treatments for neurodegenerative diseases like Alzheimer’s Dementia (AD) and Amyotropic Lateral Sclerosis (ALS), where the company’s lead candidate is Alpha-1062, a patented new chemical entity in development for AD.
ACOG announced on Thursday a private placement financing for gross proceeds of $6.8 million with an expected February 15 closing date. The financing will see ACOG offer up 26.7 million units at $0.255 per unit and each unit comprised of one common share and one share purchase warrant entitling the purchaser to one additional common share at a price of $0.39. The company said it will use the proceeds to advance its clinical programs, to complete a new NDA for ALPHA-1062 and for working capital and corporate purposes.
Commenting on the capital raise, Sarugaser wrote, “While this ~50 per cent dilution is indeed extremely painful, we take heart in the fact that the company lives to fight another day, enabling ACOG to submit its NDA to the FDA in ~2Q23, which, assuming it is approved in 1Q-2Q24, should then breathe new life into this story.”
With the “Market Perform” rating, Sarugaser has dropped his target price from $3.00 to $1.00 per share, which at press time represented a projected one-year return of 133 per cent.
“For now, we calculate in the dilution from this transaction and assume another $25 million to enable commercialization in addition to a tolerability study, which leads us to cut our target price to $1,” he said. “As such, we expect ACOG’s stock to remain range bound until FDA approval in early 2024, so we downgrade to Market Perform.”
Sarugaser provided an updated timeline projection for Alpha Cognition, saying the company should be submitting its NDA during the second quarter 2023, followed by the FDA response (ideally, an approval, Sarugaser said) in the first quarter 2024, and followed by commercialization of Alpha-1062 in the US by the third quarter 2024. After that, Sarugaser sees ACOG completing a tolerability study by the fourth quarter 2024 and then accelerated commercialization by the first quarter 2026.




 Share
Share Tweet
Tweet Share
Share




Comment