After meeting with a key opinion leader in the gastroenterology field, Raymond James analyst Rahul Sarugaser is staying bullish on biotech company Eupraxia Pharmaceuticals (Eupraxia Pharmaceuticals Stock Quote, Charts, News, Analysts, Financials TSX:EPRX). Sarugaser reiterated an “Outperform” rating on EPRX in a Monday report as well as a maintained 12-month target price of $9.00 per share.
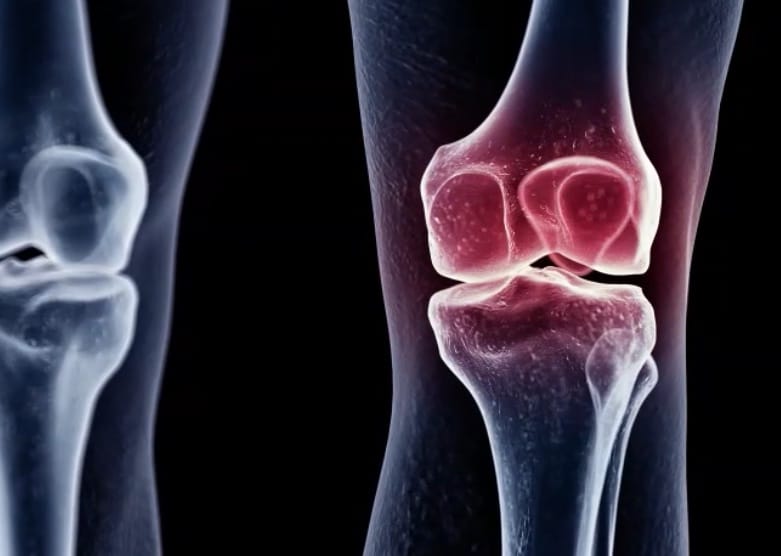
Eupraxia is a Victoria, BC-based clinical stage biotechnology company focused on locally-delivered, extended-release therapeutics. The company’s lead candidate is EP-104IAR, which is in development for the treatment of osteoarthritis of the knee.
Sarugaser recently held a call with Dr. Evan S. Dellon, Professor of Medicine and Adjunct Professor of Epidemiology at the University of North Carolina School of Medicine in Chapel Hill. Dr. Dellon is currently Director of the UNC Center for Esophageal Diseases and Swallowing (CEDAS) and has also served as an Associate Editor for Clinical Gastroenterology and Hepatology.
Sarugaser spoke with Dr. Dellon about Eupraxia’s new Eosinophilic Esophagitis (EoE) clinical program. As the analyst relayed in his report, he had four takeaways from the discussion, starting with the subject of the current standard of care treatment for EoE which involves oral viscous budesonide and injectable dupilumab, which offer “sub-optimal efficacy, sub-optimal modes of administration, or are cost prohibitive, leaving EoE as a continued high unmet need,” Sarugaser wrote.
The upshot is that steroids work for EoE but are short-lived, meaning, theoretically, that should a depot of steroid be injected deeper into the esophageal tissue, this would provide a “novel and patient-friendly therapy.”
Secondly, Sarugaser related that the discussion revealed that Eupraxia’s Phase 2 trial in EoE is “very likely to work,” according to Dr. Dellon.
Thirdly, Dr. Dellon said Eupraxia device would align well with the standard practice by gastroenterology docs, who are very familiar with scope-directed injections and would see financial benefit from higher billings for each procedure such as, for example, a scope-directed injection of EPRX’s potential EoE drug EP-104IAR.
And Fourthly, that because esophageal strictures are generally associated with EoE but are also present due to other issues such as surgery, reflux and cancer radiation, EPRX’s drug could be used off-label or have its label expanded to include other purposes.
“EoE represents a current total addressable market (TAM) of ~150,000 patients in U.S. + Canada + EU5. Addition of esophageal strictures would increase this TAM by ~50 per cent,” Sarugaser wrote.
By the numbers, Sarugaser is projected zero revenue for Eupraxia in 2022 and 2023 and expecting negative $14 million in EBITDA for 2022 and negative $12 million for 2023. At press time, Sarugaser’s $9.00 target represented a projected one-year return of 114 per cent.




 Share
Share Tweet
Tweet Share
Share




Comment