Alpha Cognition has target trimmed by iA Capital
The trial results have been positive from Vancouver-based drug developer Alpha Cognition (Alpha Cognition Stock Quote, Charts, News, Analysts, Financials TSXV:ACOG) but the company will need more funding to bring its lead asset into commercialization. That’s the take from iA Capital Markets analyst Chelsea Stellick, who reviewed the latest quarterly results from Alpha Cognition in a Tuesday update to clients.
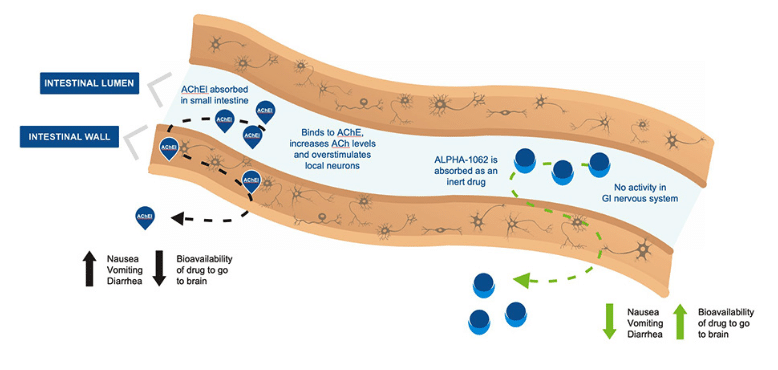
Alpha Cognition is a clinical-stage drug company focused on treatments for neurodegenerative diseases, with lead asset ALPHA-1062 in development for Alzheimer’s disease and mild traumatic brain injury. The company published its third quarter 2022 financial data on Tuesday, showing operating expenses of $2.9 million compared to $3.1 million a year earlier and a net loss of $2.1 million or $0.04 per share, and it ended the term with $3.7 million in cash compared to $11.3 million a year earlier.
ACOG announced earlier this year positive topline results from a bioequivalence study on ALPHA-1062, while it’s currently planning a RESOLVE trial to demonstrate the differentiated tolerability and titration schedule of the drug compared to other acetylcholinesterase inhibitors (AChEI). The company is preparing a new drug application on ALPHA-1062 to the US FDA on Alzheimer’s and mild traumatic brain injury.
“The Company continues to prepare its NDA filing for ALPHA-1062 for mild to moderate Alzheimer’s disease. If approved, ALPHA-1062 would be the first innovative oral therapy available for mild-to-moderate Alzheimer’s disease in over a decade. Market research shows a need for an efficacious Alzheimer’s therapy with minimal GI and insomnia-related adverse events,” said ACOG CEO Michael McFadden in a press release.
Commenting on ACOG’s financial position and timelines, Stellick said ALPHA-1062’s RESOLVE trial has completed start-up activities and should see initiation within one quarter of securing funding. Stellick noted ACOG’s recent withdrawal of a public offering, launched on November 17, with the company citing weak market conditions for the withdrawal and she said ACOG’s short cash runway into the first quarter 2023 puts a funding overhang on the stock, one which will need to be addressed either with upfront partnership fees or more creative funding options like debt.
“ACOG continues to seek additional capital to fund this study which will be crucial for differentiating ALPHA-1062 in the multi-billion-dollar AChEI market, although RESOLVE is not required for approval of ALPHA-1062,” she wrote.
“We are highly confident in the success of ACOG’s new drug application (NDA) submission to the FDA through the 505(b)(2) pathway, given the positive data obtained from the pivotal trial and follow on study that demonstrated bioequivalence to galantamine hydrobromide (Razadyne) in both the immediate and extended release formulations, respectively. NDA submission remains on track for Q2/23, ahead of commercial launch planned for early 2024,” she said.
Stellick has revised her modelling on ACOG to account for the funding overhang and has reiterated a “Speculative Buy” rating on the stock while lowering her target from C$8.00 to C$5.00, representing at press time a projected return of 1,288.9 per cent at the time of publication.
“We believe ACOG has the right asset, data, strategy, and experience to find a partner to fund RESOLVE and launch commercialization efforts for ALPHA-1062 in 2024 following what we deem as a high probability of FDA approval given the bioequivalence data previously reported,” Stellick wrote.

