Alpha Cognition is heading to $4 per share, says Raymond James
Raymond James analyst Rahul Sarugaser is bullish in his support of Alpha Cognition (Alpha Cognition Stock Quote, Chart, News, Analysts, Financials TSXV:ACOG), maintaining an “Outperform 2” rating while raising his target price from $3.50/share to $4/share for a projected return of 556 per cent in a recent update to clients.
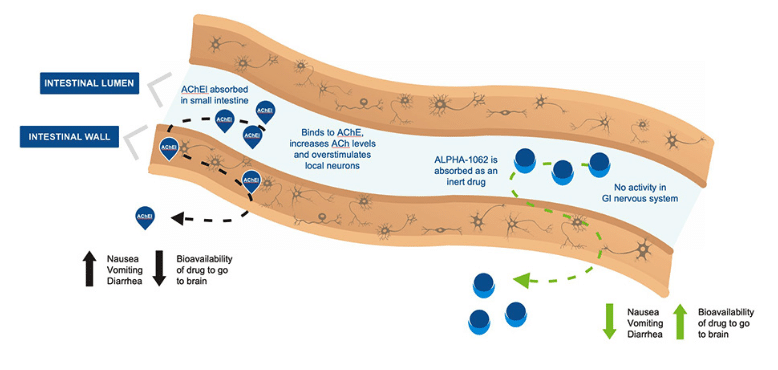
Vancouver-based Alpha Cognition is a clinical stage biopharmaceutical company dedicated to developing treatments for neurodegenerative diseases like Alzheimer’s Dementia and Lou Gehrig’s Disease, with its lead candidate being the Alpha-1062, an acetylcholinesterase inhibitor (“AChEI”) for the treatment of Alzheimer’s disease with minimal gastrointestinal side effects.
Sarugaser’s latest update on June 22 came after the company announced strong results from its pivotal bioequivalence study of ALPHA-1062, which is a prodrug formulation of galantamine for the treatment of mild to moderate Alzheimer’s Disease.
“Top-line results confirmed that ALPHA-1062 achieved bioequivalence: equivalent pharmacokinetics (peak exposure [Cmax], total exposure [AUC]) with standard-of-care galantamine hydrobromide, and, critically, no adverse events were reported among the 72 patients treated (n=36 drug, n=36 placebo),” Sarugaser said. “ACOG plans to submit an NDA to the FDA for ALPHA-1062 in mild to moderate AD with these data during 2Q23 (once the company is satisfied with its CMC preparations), estimating a 1Q24 FDA approval. Should ALPHA-1062 be approved, it would be the first new market entrant in AD in over a decade.”
Following the success of the bioequivalence study, Sarugaser notes that the company’s next move will be to launch a patient tolerability trial of ALPHA-1062, which will be called RESOLVE, in the third quarter of 2022. The trial will be designed to evaluate safety and tolerability of both a standard-titration dosing arm and a fast-titration dosing arm against a placebo; Sarugaser also notes that final trial design and patient numbers have not yet been published.
“Should ALPHA-1062 demonstrate ‘placebo-like’ levels of overall adverse events (AEs) and specific AEs (diarrhea, nausea, vomiting, insomnia, dizziness)—similar to the result of the present BABE trial—this would be game-changing,” Sarugaser said.
Sarugaser pointed to a 2012 placebo-controlled trial of galantamine hydrobromide among 2,045 patients, which yielded a 54 per cent rate of total treatment-emergent AEs, which has led to a significant majority of the AD population (Sarugaser suggested between 80 and 90 per cent) failing first-line therapy treatment.
Sarugaser also noted the particular importance of the fast-titration option, given that adverse effects with present treatment standards (dose schedules between six and 12 weeks) have been happening before reaching the point of an effective dosage and any meaningful clinical benefits.
With the fast-titration option, Alpha Cognition is seeking to cut that time down significantly as part of the RESOLVE trial, as it will assess the safety and tolerability of reaching a therapeutic dose level within two weeks instead of two months, which Sarugaser notes to be pivotal for a drug that could provide clinically meaningful symptom improvements to the Alzheimer’s patient population in the United States, which stood at approximately 5.8 million in 2020 and forecasts to grow to 8.4 million by 2030, then jumping to an estimate 13.8 million by 2050.
“ACOG aims to prove that ALPHA-1062 is well-tolerated in the context of a fast-titration dosing regimen, which could motivate the FDA to reflect this in its label post-approval, thus providing further motivation for physicians to recommend ALPHA-1062 to patients as a second-line (or first-line) therapy,” Sarugaser said.
Following completion of the RESOLVE trial, Sarugaser forecasts an NDA filing with the U.S. Food and Drug Administration in the second quarter of 2023, with an expectation of receiving marketing clearance early in the first quarter of 2024 ahead of an official launch at the end of the same quarter.
“We are very pleased with the positive results from the current studies of our lead asset, ALPHA-1062,” said Cedric O’Gorman, MD, Chief Medical Officer of Alpha Cognition in the company’s June 22 press release. “If approved, ALPHA-1062 could provide a meaningful advancement for patients with Alzheimer’s Disease, especially those who are unable to tolerate the gastrointestinal side effects that often occur with many of the current treatment options.”
Sarugaser maintained his overall financial projections for the company, as he forecasts a loss of $7 million in EBITDA for 2022 before turning positive at a projected $7 million for 2023, with no revenue report in that timeframe.
“There is a significant need for innovative new treatment options for patients living with Alzheimer’s Disease, as the currently available medicines offer limited symptomatic relief,” added Dr. James Galvin, MD, Professor of Neurology, Chief of the Division of Cognitive Neurology, and Director of the Comprehensive Center for Brain Health at the University of Miami in the June 22 release. “If approved by the FDA, ALPHA-1062 could provide an exciting next-generation treatment option for patients living with Alzheimer’s Disease.”
Alpha Cognition’s stock price has fallen by about 50 per cent over the course of 2022, gradually falling off after hitting an early peak of $1.13/share on January 18 and closing at a 2022 low of $0.55/share on Monday.

