Research Capital Corporation analyst Andre Uddin is maintaining his “Hold” rating on Profound Medical (Profound Medical Stock Quote, Chart, News TSX:PRN) after the company’s latest quarter. In an update to clients on Wednesday, Uddin said PRN’s second quarter results were mixed but there are signs of a return to form for the company’s TULSA-PRO medical device.
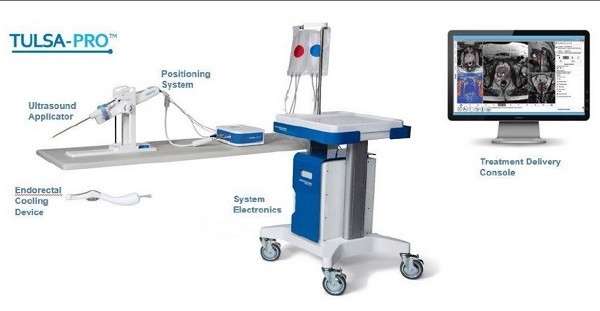
With its headquarters in Mississauga, Ontario, Profound Medical is commercializing a novel noninvasive, image-guided therapeutic technology, the TULSA-PRO, which combines real-time magnetic resonance imaging with transurethral, robotically-driven therapeutic ultrasound and closed-loop thermal feedback control. The system is designed to provide precise ablation of pathologic prostate tissue (including prostate cancer and BPH) while simultaneously protecting critical surrounding anatomy.
Profound Medical’s Q2 revenue of $2.6 million ($1.5 million in capital sale + $1.2 million in recurring revenues) was in line with Research Capital’s projection of $2.6 million as well as being slightly ahead of the consensus estimate of $2.2 million. The revenues almost marked a 270 per cent increase over the $1 million in revenues for this quarter last year. PRN had a Q2 net loss of $7.0 million or $0.35/share versus a loss of $5.3 million or $0.33 per share a year earlier, with the consensus and Profound estimates for the second quarter both being negative $5.9 million or $0.29/share. (All figures in US dollars except where noted otherwise.)
Uddin said Profound will be commercializing TULSA-PRO through three channels: early adopters (private urology practices), independent imaging centres and large teaching hospitals. All told, systems have been installed at 14 sites across all three channels, with ten currently operational and a management expectation of 25 sites having TULSA-PRO installed by the end of the year. Initial locations and installations for the technology include the Memorial Hermann Health System in Houston, Methodist Hospital in San Antonio, and the Mayo Clinic in Rochester, Minnesota.
In addition, Profound its putting its TULSA technology against the process of radical prostatectomy to evaluate safety, efficacy, and non-inferior progression-free survival as the company continues a CPT-1 Code application for its product, with the optional study acting to potentially further support payer coverage and drive widespread adoption.
The company believes it will have sufficient clinical data by the end of this year to file for the CPT-1 code by the first half of 2022, as well as a ruling from the American Medical Association for reimbursement before the end of 2022, with Uddin noting that the code becoming effective in January 2024 would significantly accelerate TULSA-PRO’s sales growth.
“The U.S. TULSA-PRO business rebound that started in March continued through the second quarter, driving a 145 per cent sequential increase in recurring revenues over the previous quarter,” said Arun Menawat, Profound’s CEO in the company’s August 4 press release. “Moving forward, while we remain cautious about the continuing impact of COVID-19, particularly in select international markets such as China and Japan, we believe that our overall Q2 financial performance bodes well for the second half of 2021.”
Uddin said the TULSA-PRO should have a wide application for numerous subgroups of prostate cancer patients, with a specific analysis on patients who have undergone TULSA-PRO procedure showing that 86 per cent had more than 50 per cent of their prostate gland tissue removed, 63 per cent had the whole gland removed, 28 per cent had a partial removal, 6 per cent were benign prostate hyperplasia (BPH) only and 1 per cent were on salvage treatment.
In terms of cancer risk in the patients, 11 per cent were considered low-risk, 53 per cent were low-intermediate risk, 28 per cent were high-intermediate risk and eight per cent were seen as high risk.
From a financial standpoint, Uddin has forecast the company to continue ramping up sales, projecting revenues of $16.1 million in 2021, then more than doubling to a projected $33.8 million for 2022, $47.9 million for 2023, $63.8 million in 2024, then climbing to $71.4 million for 2025, which is also the first year Uddin forecasts positive values for earnings per share and cash flow per share.
Uddin also foresees particular data multiples coming into clearer focus, with P/Sales dropping to a projected 17.3x in 2021 from 38x in 2020, then down to 8.2x in 2022, 5.8x in 2023, 4.3x in 2024 before dropping to 3.9x by 2025.
The EV/Sales multiple appears to follow a similar path, falling from 27.9x in 2020 to a projected 12.7x in 2021, dropping off heavily to 6x in 2022, 4.2x for 2023, 3.2x in 2024 before hitting 2.9x by 2025.
In spite of the potential rebound and the prospect of third quarter results coming in November, Uddin believes Profound is still a wait-and-see prospect. With the update, Uddin has maintained his target of C$21.50, which at the time of publication represented a projected one-year return of 19 per cent.
“We are lowering our gross margin assumption for Q3 and increasing R&D expense estimates for 2021,” he said. “We are maintaining our HOLD rating as we await better visibility on U.S. sales ramp-up of TULSA-PRO.”
PRN has had a dramatic up and down over the past 12 months, going from C$21.00 last August to as high as C$35.00 by early February. Since then it’s been mostly downhill, and the stock is currently back around the C$20.00 mark.
Comment
One thought on “Take a pass on Profound Medical, says Research Capital”
Leave a Reply
You must be logged in to post a comment.




 Share
Share Tweet
Tweet Share
Share




How many procedures were performed in the last quarter and YTD?