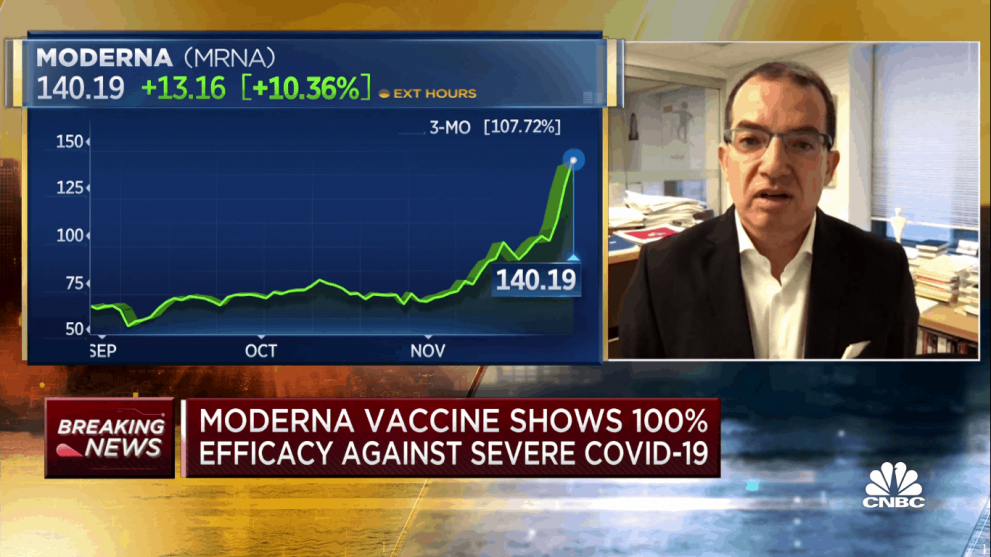
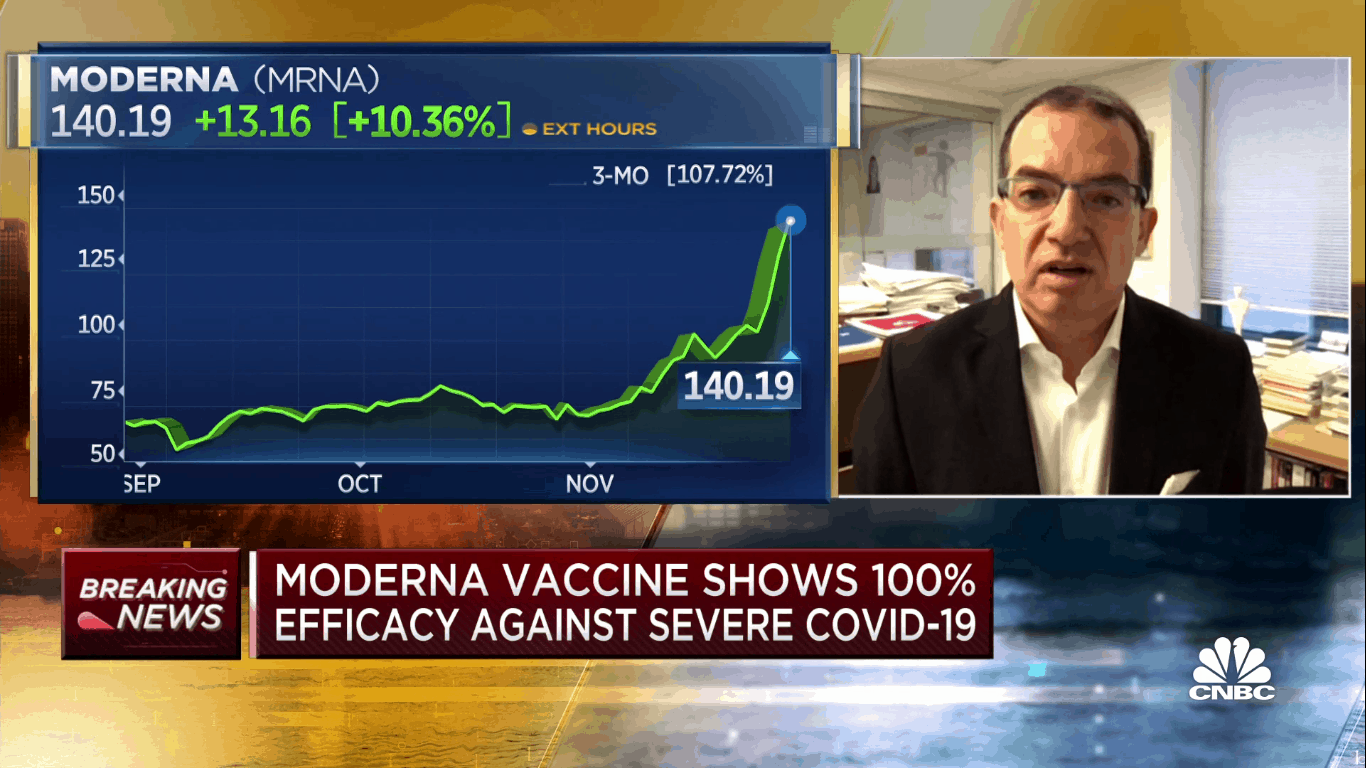
Pfizer and AstraZeneca made their announcements over the past few weeks and now it’s Moderna’s turn, coming out on Monday with primary data from a Phase 3 trial of its coronavirus vaccine candidate which has proven 94.1 per cent effective in preventing people from the disease.
The COVE study involved more than 30,000 participants in the US, with the results showing 196 cases of COVID-19, 185 of which were observed in the placebo group versus 11 in the group inoculated with mRNA-1273. Moderna says it will now request an emergency use authorization from the US Food and Drug Administration and conditional approval from the European Medicines Agency.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and importantly, the ability to prevent severe COVID-19 disease. We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations and death,” said Stéphane Bancel, CEO of Modern in a press release.
Moderna’s share price vaulted ahead on Monday, climbing 20 per cent on the news. That puts the stock at more than a double for the month of November and gives it a year-to-date return of 685 per cent.
Bancel said the 100-per-cent efficacy in preventing severe cases of COVID-19 was more than encouraging.
“The most exciting news to me yesterday when I learned the data was the severe cases, because if we can prevent severe cases it means reduced hospitalization and no death, and that will be, I believe, a game-changer in this pandemic,” said Bancel, speaking on CNBC on Monday.
Bancel said some questions have yet to be answered about Moderna’s vaccine, including for how long it will be effective and whether it offers complete protection from contracting the coronavirus. Bancel said they could be still months away from figuring that out.
“We still need a bit more time to go over sensitive data to understand the duration of the vaccine,” Bancel said. “We have much more data on neutralizing antibodies, which, as this Phase 3 data shows is very predictive to efficacy. And so I think we should be able to get a good sense of a shape of a curve to give us an idea of duration, in terms of preventing infection. We need a bit more data. I hope in a couple of months we should have it.”
Moderna says it will be able to produce between 500 million and a billion doses of mRNA-1273 over the course of 2021. For its part, Canada made an agreement with Moderna in August to guarantee 20 million doses with an option to get an additional 36 million. The United States is reportedly on record for up to 500 million doses.
Beyond its coronavirus vaccine, Bancel said Moderna’s pipeline is looking strong, with four programs in Phase 2 studies, including its CMV (Cytomegalovirus) vaccine which had positive interim data earlier this year.
“We have six more vaccines in development,” said Bancel. “We’re going to start another Phase 3 in 2021 against CMV cytomegalovirus. It’s the number one cause of birth defects in the United States. 10,000 kids per year are born with birth defect because the mom got infected during pregnancy and there is no vaccine approved on the market.”
“I believe we have a very high chance, now that we know this platform is providing strong efficacy, to have potentially a CMV vaccine launch as well — and that vaccine we believe is $2 to $5 billion of annual peak sales,” Bancel said.
Moderna is also planning on putting a flu vaccine to trials, aiming to improve on the average in current vaccines of between 50 and 60-per-cent efficacy.
“We think the world needs a better flu vaccine, and we are going to work really hard to get that to work,” Bancel said.
Moderna, which launched as a public company in 2018 with the largest-ever biotechnology IPO, last reported financials in late October, where it posted total revenue of $157.9 million for its third quarter compared to $17.0 million a year earlier. The jump in revenue came from increases in grant revenues. Research and development expenses for the quarter were $611.5 million and the company posted a net loss of $233.6 million for the quarter.
With the COVID-19 vaccine coming, 2021 should be “the most important inflection year in Moderna’s history,” according to Bancel, from the October 29 press release.
Leave a Reply
You must be logged in to post a comment.



 Share
Share Tweet
Tweet Share
Share




Comment