 Raymond James analyst Rahul Sarugaser thinks Profound Medical Corp (Profound Medical Corp Stock Quote, Chart, News TSX:PRN) is profoundly undervalued.
Raymond James analyst Rahul Sarugaser thinks Profound Medical Corp (Profound Medical Corp Stock Quote, Chart, News TSX:PRN) is profoundly undervalued.
In a research report to clients today, the analyst initiated coverage of the Toronto-based medical device company with an “Outperform” rating and a one-year price target of $4.00, implying a return of 339.6 per cent at the time of publication.
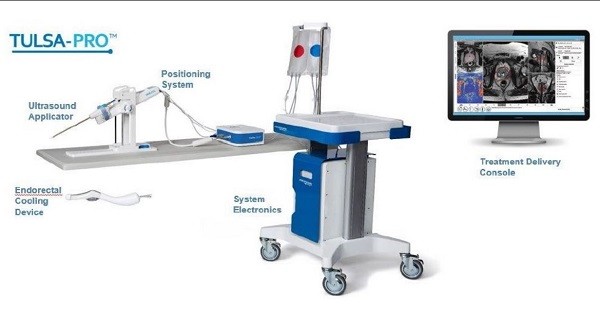
Sarugaser says Profound, which is commercializing a novel technology called TULSA-PRO fo the treatment of prostate cancer, has a compelling clinical value proposition.
“PRN’s Phase III/Pivotal trial data showed that TULSA-PRO is able to ablate either the entire prostate or specific focal regions, all while preserving the sensitive nerve bundles that are in close proximity to the organ,” the analyst writes. “This safety profile has resulted in a marked improvement in the adverse outcomes seen in current standard of care—surgical prostatectomy or radiation—significantly reducing erectile dysfunction (from ~65% to ~20%) and urinary incontinence (from ~15% to ~2%). As such, patients with intermediate “GG2” disease, for whom urologists aim to balance the risk of advancing disease with the adverse outcomes of incontinence or impotence, now have a better treatment option with at least equal efficacy, but a far better safety profile. This, we believe, will be a primary driver of adoption of TULSA-PRO over the next three years, establishing it as the standard of care for treatment of intermediate prostate cancer.”
Sarugaser thinks the company will post EBIT of negative $19.1-million on net revenue of $12.1-million in fiscal 2020. He expects those numbers will improve to EBITDA of negative $11.1-million on net revenue of $27.3-million in fiscal 2020.
The analyst says he estimates imply a modest penetration rate for PRN.
“We use a conservative 1.25% penetration rate during the first three years of TULSA-PRO’s commercialization, while revenues will still be patient-pay (i.e., pre-reimbursement),” he notes. “For these years—2020, 2021, and 2022—we estimate revenues of $12 mln, $27mln, and $54 mln respectively. We view these as very achievable revenue estimates because by 2022 an installed base of just 110 devices, each treating 110 patients per year, would generate $46 mln in revenue from TULSAPRO, plus an additional $8 mln from ancillary products and services.”
“Our $4.00 price target is based on a 5-year discounted cash flow analysis—using a discount rate of 10%, and a terminal rate of 2%—yielding a total valuation for PRN of $436 mln, or $4.03 per share (rounded to $4.00), a >350% premium to PRN’s current share price, Sarugaser adds.
On August 16, Profound Medical announced it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market Tulsa-Pro for ablation of prostate tissue.
“We are pleased with the FDA’s expeditious review of our application,” said CEO Dr. Arun Menawat. “We believe this, combined with the label that the agency approved for Tulsa-Pro, serves as a testament to our technology’s strong clinical profile. While we have been conducting a limited commercial launch of Tulsa-Pro in select CE mark jurisdictions over the past few quarters, in many respects, we only just crossed the starting line following the recent reporting of the positive TACT trial clinical results and today’s announcement of FDA 510(k) clearance. The feedback from physicians using the system in Europe, and from key opinion leaders in the United States who have first-hand experience with the technology as TACT trial investigators, has been very positive, particularly regarding the ease of use, efficiency and flexibility based on patient needs; in addition to its excellent patient tolerability and short recovery periods. Given this interest and feedback, we are very much looking forward to working with our strategic partners, Philips and Siemens Healthcare, in concert with our direct sales and marketing teams, to prepare for the U.S. commercial launch of Tulsa-Pro in the fourth quarter of 2019.”
Leave a Reply
You must be logged in to post a comment.




 Share
Share Tweet
Tweet Share
Share




Comment