Toronto's Xagenic Inc. completes successful beta testing of X1 diagnostic system

 Toronto-based developer of lab-free molecular diagnostics platform Xagenic Inc. has announced that the Beta version of its fully automated X1 molecular diagnostic testing system has undergone successful testing at an external clinical site.
Toronto-based developer of lab-free molecular diagnostics platform Xagenic Inc. has announced that the Beta version of its fully automated X1 molecular diagnostic testing system has undergone successful testing at an external clinical site.
The external study confirmed the feasibility of point-of-care testing using the X1 system, and that the CT/NG (combined chlamydia and gonorrhoea) test was able to generate results comparable to gold-standard molecular tests normally run at centralized labs.
“We are very excited about the progress of the X1 testing system and demonstrating its clinical utility during Beta testing,” said Xagenic COO Randall Wilhoite. “The information we have collected will inform the completion of the final design for the X1 and the development of menu for this first-in-class POC molecular testing system.”
Successful conclusion of this Beta testing precedes Xagenic’s plans for market launches in Europe and the U.S. in the near future.
Xagenic’s X1 system is a cartridge-based, point-of-care platform to test for infectious diseases, such as flu, strep and upper respiratory infections, although this first assay developed for the X1 system is a combined chlamydia and gonorrhoea (CT/NG) test designed to facilitate screening, diagnosis and rapid treatment of these common sexually-transmitted diseases.
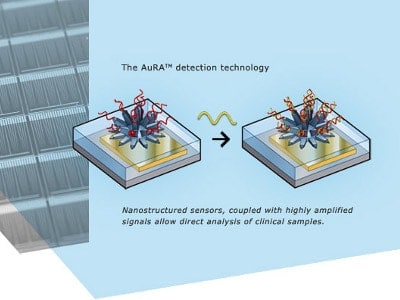
The single-use cartridge system contains a proprietary sensor chip that enables direct detection of chlamydia and gonorrhea without the use of enzymes, and is an electrochemical amplification-based technology protected by 17 patent families globally.
The X1 system is intended to deliver a definitive test result during the time available during a typical on-site office consult with a physician, typically requiring less than a minute of physician hands-on time running with the device.
Normally, tests require that samples to be sent to centralized labs before results are obtained, a process that can last anywhere between two to seven days.
Bringing rapid on-demand testing to physician offices eliminates costs associated with that out-sourced transportation and testing infrastructure, while also improving patient compliance and outcomes, and reducing complications and unnecessary direct costs.
In 2015, Xagenic announced a $15 million round of financing, led by investors that had previously participated in its 2014 $25.5 million Series B round of funding, including Domain Associates, CTI Life Sciences, BDC Capital and the Ontario Capital Growth Corporation.
Between that and Xagenic’s $10 million in Series A funding, the company has raised $52.7-million in financing to date.
Xagenic has origins in the University of Toronto, and was launched in 2010 with $1 million seed money from MaRS Innovation, the Ontario Institute for Cancer Research, the Health Technologies Exchange, and the Ontario Centres of Excellence.
In 2015, Xagenic acquired exclusive rights to a mutation detection technology, developed by Dr. Shana Kelley, whose University of Toronto team has developed a close working relationship over the years with Xagenic.
That team published a study on the technology in Nature Chemistry, outlining its capacity to detect cell-free nucleic acids in blood plasma or serum, meaning that Xagenic’s platform would be able to run a kind of “liquid biopsy” or deliver results without taking a tissue sample.
Headquartered in Toronto, Xagenic also has a San Francisco office.

Terry Dawes
Writer