Roth Capital Partners analyst Kumaraguru Raja likes the look of biotech stock Genenta Science (Genenta Science Stock Quote, Charts, News, Analysts, Financials NASDAQ:GNTA), saying in a Thursday transfer of coverage report that initial data from a Phase 1/2 cancer trial is promising.
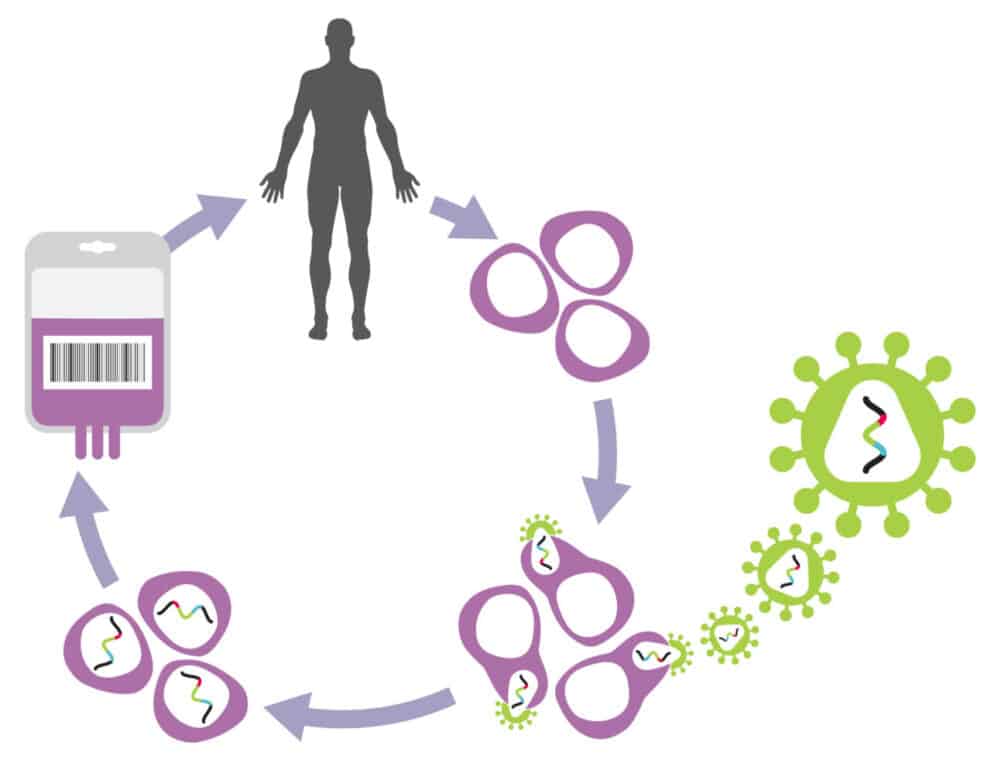
Developmental-stage biotechnology company Genenta has a cell-based therapeutic platform using tumour-associated monocytes known as TEMs or Tie-2 monocytes, which have the ability use tumour-directed angiogenesis against the tumour itself by delivering immunomodulatory cells directly into the tumour through its own network of blood vessels. Because of the role of angiogenesis in the proliferation of tumours, this process has potential applicability across a broad variety of solid tumours.
The company’s lead clinical candidate is Temferon, which uses a selective delivery of its interferon-alpha payload directly to the tumour, thereby avoiding potential off-target effects and systemic toxicity. Currently in a Phase 1/2a open-label, multicentre trial in glioblastoma patients, temferon has Orphan Drug Designation (ODD) with the US FDA and the European Commission for the treatment of patients with glioblastoma multiforme.
Raja said he expects the ODD status to accelerate the development of temferon and noted that ODD leads to ten years of market exclusivity upon receiving marketing authorization in the EU and seven years of exclusivity following biologics license application (BLA) approval in the US.
“We believe that Temferon’s targeted approach to treat glioblastoma without off-target effects and systemic toxicity will potentially lead to longer overall survival and better quality of life. Temferon has potential in multiple solid tumors as well as hematological cancers based on pre-clinical data,” Raja wrote.
“Genenta’s technology utilizes the tumour homing ability of naturally occurring proangiogenic Tie-2 monocytes (TEMs) to deliver a cytokine interferon-alpha (IFN-α) precisely to tumours. Temferon Phase 1/2 trial in glioblastoma is ongoing, and the initial data is promising, in our opinion. Temferon has potential in multiple solid tumours and hematological cancers based on pre-clinical data. We anticipate significant potential for the platform as the pipeline advances,” he said.
In his financial model for Genenta, Raja is projecting temferon approval and launch in glioblastoma in 2027, with peak market share of 20 per cent and peak sales of $1.15 billion in 2039.
Up ahead, Raja said selection of a second indication for temferon is expected in the second half of 2023, while cohort 7 and 8 data is expected by the year end and conclusion of patient enrolment and treatment administration is expected in the first quarter 2024.
Raja gave Genenta a “Buy” rating and $15.00 target, which at press time represented a one-year projected return of 155 per cent.


 Share
Share Tweet
Tweet Share
Share


Comment