Shares of Arch Biopartners (Arch Biopartners Stock Quote, Charts, News, Analysts, Financials TSXV:ARCH) have been jumping the past couple of trading sessions after the US FDA green-lit a new clinical trial for the biotech company.
But even with the good news, Raymond James analyst Rahul Sarugaser is sticking with a “Market Perform” rating on the stock, saying in a Tuesday update that caution is the wise approach, at least until further clinical results arrive.
Toronto-based Arch Biopartners, which is developing new drug candidates for inflammation-related conditions, with lead candidate Metablok/LSALT peptide, announced on Tuesday that it has received a Study May Proceed letter from the FDA regarding its planned Phase 2 human trial with LSALT, targeting cardiac surgery-associated acute kidney injury (CS-AKI).
The trial will involve about 240 patients with CS-AKI, a condition for which no treatments exist for prevention or treatment. Arch said the trial is to begin recruitment in the fall of 2023.
“The FDA’s decision to grant a Study May Proceed letter is the culmination of 18 months of work by the Arch team to scale up LSALT manufacturing, perform dose escalation studies for LSALT peptide and complete significant planning and preparation for the CS-AKI trial,” said Arch CEO Richard Muruve in a statement.
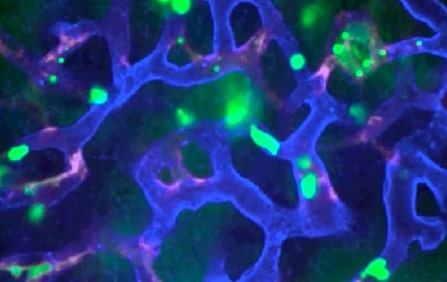
Arch is pursuing LSALT peptide’s clinical development to treat acute injury in the lungs, kidneys and liver caused by inflammation, with the drug pathway being to inhibit DPEP-1, an enzyme that transports inflammatory agents to organs. A team at the University of Calgary invented LSALT peptide, with the findings of its pathway in inhibiting neutrophil-driven inflammatory diseases published in the journal Cell in 2019.
Arch’s share price has climbed 16 per cent combined on Monday and Tuesday to hit $2.30 per share, which is still considerably off from the $4.80 highs hit a year and a half ago.
For Sarugaser, he is maintaining a 12-month target of $3.50, which at the time of his report’s publication represented a projected return of 64 per cent.
“We remain cautiously optimistic as we await publication of ARCH’s Ph 2 COVID data (est. 2H23)—which, in our view, will be seminal in determining the likely success (or failure) of this upcoming Ph 2 trial in CS-AKI—before making any further prognostications on its predictiveness, so we maintain our Market Perform rating,” Sarugaser wrote.
Sarugaser said conversations between Raymond James and key opinion leading (KOL) researchers and clinicians familiar with LSALT peptide have said that the drug has the potential to be a breakthrough therapy in the prevention, treatment and recovery process from AKI, among other indications.
“Patients frequently avoid cardiac and other surgeries—and physicians warn against them—because of certain populations’ elevated risk associated with AKI; KOLs suggest that an AKI-specific therapy would be a game-changer in reducing the downside risk of surgery,” he said.


 Share
Share Tweet
Tweet Share
Share


Comment