Appili Therapeutics (Appili Therapeutics Stock Quote, Chart, News TSX:APLI) isn’t completely suiting Douglas Loe’s eye these days, as the Leede Jones Gable analyst maintained a “Hold” rating and target price of $0.25/share for a projected return of 92 per cent in an update to clients on Monday.
Halifax-based Appili Therapeutics acquires and develops novel medicines to address unmet needs in the infectious disease sector, including influenza drug Favipiravir, the antifungal candidate ATI-2307 for treating cryptococcal meningitis and invasive candidiasis, the ATI-1503 offering that develops a class of gram-negative targeting antibiotics, and its ATI-1501 liquid oral suspension formulation of metronidazole.
Loe’s latest analysis comes after Appili announced that the U.S. Department of Defense, via the Joint Science and Technology Office of the Defense Threat Reduction Agency, would provide Appili with over US$10 million in R&D capital to fund ongoing preclinical testing of its live, attenuated virus formulation ATI- 1701, targeting the biodefense-relevant respiratory bacterial pathogen Francisella tularensis.
Loe views the news positively, though his projections don’t presently model value for the antimicrobial asset.
“There is obviously no downside to receiving non-dilutive R&D grant capital to fund pipeline development, especially when the magnitude of the grant provides substantial capital to achieve a seminal regulatory milestone, in this case an eventual IND filing,” Loe said. “Moreover, the capital infusion from a major US government department provides us with supporting evidence that ATI-1701 is seen by a third party as being relevant to expanding the country’s biodefense capabilities, and that ATI-1701 is an attractive, if early-stage, candidate for mitigating widespread Francisella tularensis infection.”
According to the company, the new funding is intended to replace and expand upon a prior contract awarded to one of Appili’s development partners, a US$6.3 million award to Ology Bioservices from October 2020. Under the terms of the new funding, Appili will serve as prime contractor and oversee a comprehensive development program for ATI-1701 that includes nonclinical, manufacturing, and regulatory activities to support an IND submission to the U.S. Food and Drug Administration.
All told, the extra capital infusion pairs with the $9.4 million in cash on hand the company at the end of its third fiscal quarter to give the company $22 million in pro-forma cash, which Loe believes his should be sufficient to substantially fund ongoing preclinical ATI-1701 testing while in parallel allowing Appili to move its ATI-2307 testing from Phase I pharmacokinetic testing to Phase II, which Loe believes should happen by the end of the calendar year.
“We are grateful for the continued support from DTRA to help us further develop ATI-1701 and deliver on our mission to address a variety of urgent unmet needs in infectious disease,” said Dr. Armand Balboni, CEO of Appili Therapeutics in the company’s February 28 press release. “Advancing ATI-1701 could have a transformative impact on mitigating this high risk to national security and public health. We look forward to continuing to advance this vaccine candidate and further strengthening our partnerships with government agencies around the world to address this urgent bioterrorism threat.”
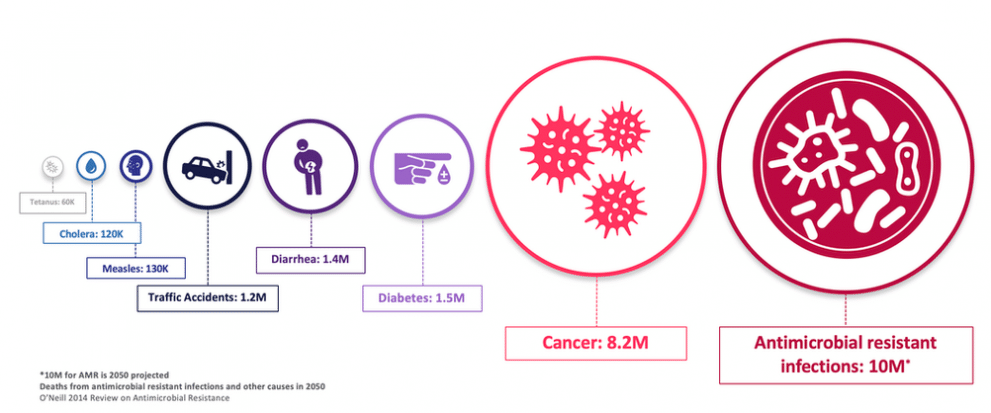
Francisella tularemia’s origins date back to the 1910s, particularly with squirrels in California, and while its threat to human health is modest at best, Loe believes the bioterrorism threat the bacteria species could present is enough to warrant continued development of treatments, which are currently limited to broad-spectrum antibiotics like streptomycin or doxycycline and other tetracycline analogs.
“The bacterium does not seem to be transmissible from human-to-human, though its comparatively low case prevalence mitigates this mode of transmission anyway, and at present there are no vaccines or immune therapies available for targeting this pathogen, making ATI-1701 the highest-profile immune therapy targeting Francisella even though it is still only in preclinical testing,” Loe said.
After estimating revenue of zero for 2021, Loe forecasts Appili bringing in $256,000 in revenue in 2022 from sales of its ATI-1501 offering, which grows gradually to a projected $784,000 in 2025. Loe starts factoring in revenue from Appili’s ATI-2307 offering in 2026, resulting in a jump to $28.2 million that year and starting a growth ramp Loe projects to reach $79.7 million by 2030.
Meanwhile, after projecting eight-figure losses through 2025, including $16.8 million losses in both 2024 and 2025, Loe forecasts the company’s EBITDA to turn positive at $13 million in 2026 for a 46 per cent margin, which he forecasts to grow to $71.3 million and an 89 per cent margin by 2030.
With an unsuccessful PRESECO trial for Favipiravir serving as a major factor, Appili’s stock price has plummeted by 88.5 per cent over the last 12 months with a 52-week low of $0.10/share coming on December 17, though it has gone back up by 4.2 per cent since the start of 2022.
“APLI share value (and our investment thesis) continues to be constrained by the shockwaves generated by negative Phase III COVID-19 infection/hospitalization data from the now-concluded 1,231-patient PRESECO trial for licensed small-molecule RNA polymerase drug favipiravir,” said Loe. “We are maintaining our HOLD rating and $0.25 PT on the stock until we have greater transparency on clinical performance of Appili’s two leading anti-microbial pipeline drugs in ATI1701 and antifungal small-molecule drug ATI-2307.”
Leave a Reply
You must be logged in to post a comment.





 Share
Share Tweet
Tweet Share
Share




Comment