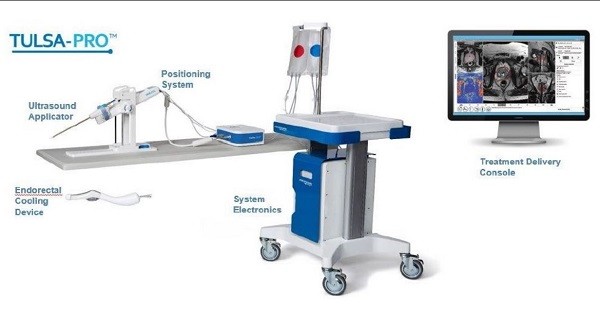
 Canadian regulatory approval for its TULSA-PRO medical device is likely to yield more international approvals for Profound Medical Corp (Profound Medical Corp Stock Quote, Chart, News TSX:PRN), says Raymond James analyst Rahul Sarugaser.
Canadian regulatory approval for its TULSA-PRO medical device is likely to yield more international approvals for Profound Medical Corp (Profound Medical Corp Stock Quote, Chart, News TSX:PRN), says Raymond James analyst Rahul Sarugaser.
The analyst reviewed the company’s latest announcement in a brief to clients on Tuesday where he reiterated his “Outperform 2” rating.
Toronto-based Profound on Monday announced that it had received approval from Health Canada for the TULSA-PRO for the ablation of low to intermediate risk organ-confined prostate cancer. The TULSA-PRO will have specialty pharma company Knight Therapeutics as its distributor in Canada.
“The positive Health Canada decision is key to our global expansion strategy for TULSA-PRO, as many major market jurisdictions, such as China, have a ‘country of origin’ approval requirement for medical devices,” said Goldy Singh, Profound’s VP, Regulatory Affairs & Product Management, in a press release.
Commenting on the event, Sarugaser said Knight Therapeutics’ royalty rate is likely to be between 20 and 40 per cent and that Profound will be responsible for assisting and training Knight’s Canadian sales team, likely commencing in Q1/2020.
Sarugaser says that revenue shouldn’t be expected to follow from the Health Canada ruling until at least a year’s time.
“One key benefit of this arrangement is that Knight Therapeutics is very adept at securing reimbursement for its marketed drugs. So, we’d anticipate it taking around two years for Knight to achieve full reimbursement in Canada, pretty much in parallel with PRN’s US reimbursement timeline,” Sarugaser says.
“One final benefit is that certain jurisdictions such as China, where PRN still seeks regulatory approval, prioritize their domestic approval based on ‘home country’ approval. As such, this Health Canada nod is strategically beneficial for PRN, and paves a path toward broader international approvals for TULSA-PRO,” Sarugaser writes.
Sarugaser thinks that Profound will have an EBITDA loss of $15 million on revenue of $5 million in fiscal 2019 and an EBITDA loss of $16 million on a top line of $12 million in fiscal 2020.
Profound reported its third quarter 2019 results on November 7, recording revenue of $682,000 and a net loss of $6.3 million or $0.57 per common share versus $5.1 million or $0.48 per common share a year earlier, with the increase being attributed to larger R&D expenses, G&A expenses and net finance costs.
Recent corporate highlights from Profound included the selling its first TULSA-PRO system in Japan, the receipt of 501(k) clearance from the US FDA to market the TULSA-PRO (with the launch expected in Q4/2019) and a ten-to-one share consolidation on October 16 in anticipation of the company’s listing on the Nasdaq exchange, which started on October 29.
“Since the recent receipt of FDA clearance to market TULSA-PRO in the United States, our main focus has been on commercialization and we have already visited some 75 US institutions,” said Arun Menawat, Profound’s CEO, in the quarterly press release.
“We are very encouraged by the resounding interest in adopting the technology, such that we expect to have a first commercial TULSA-PRO site operational and treating patients before year-end,” said Menawat.
Leave a Reply
You must be logged in to post a comment.


 Share
Share Tweet
Tweet Share
Share




Comment