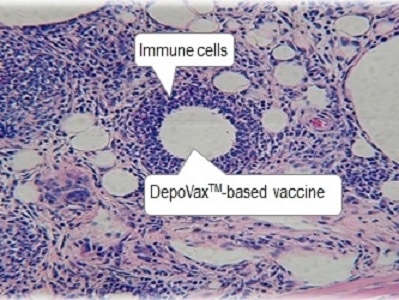
 Mackie Research Capital analyst Andre Uddin says Immunovaccine Inc.’s (Immunovaccine Inc. Stock Quote, Chart, News: TSX:IMV) interim results for its phase 1b clinical study of its novel T-cell-activating immuno-oncology candidate, DPX-Survivac, are “what you want to see for efficacy”.
Mackie Research Capital analyst Andre Uddin says Immunovaccine Inc.’s (Immunovaccine Inc. Stock Quote, Chart, News: TSX:IMV) interim results for its phase 1b clinical study of its novel T-cell-activating immuno-oncology candidate, DPX-Survivac, are “what you want to see for efficacy”.
This morning, Immunovaccine released the first interim data analysis from its continuing phase 1b clinical study of its novel T-cell-activating immuno-oncology candidate, DPX-Survivac, in combination with epacadostat and low-dose cyclophosphamide. The company said that based on the interim analysis, the combination therapy appears to have an acceptable safety profile.”
“We are very encouraged by these early data, which are tremendously important to ImmunoVaccine, as they help to validate the underlying clinical potential of DPX-Survivac,” said CEO Frederic Ors. “Research is consistently demonstrating that activating T-cells is a crucial mechanism to improving tumour response rates. This desired mechanism of action is exactly what we have developed DPX-Survivac to address, and this data set has provided an encouraging first clinical demonstration of this effect.”
Uddin says these results are promising.
“The interim results involving the first 4 patients enrolled show that the combined use of DPX-Survivac and Epacadostat was safe with no severe adverse events (SAE) reported (1 patient had a grade 3 and 1 patient grade 4 safety -primarily due to rash),” notes the analyst. “T cell infiltration in tumors in 3 of the 4 patients based on RNA sequencing following treatment was also observed, which validated the mechanism of action of DPX-Survivac and Epacadostat. Immune checkpoint markers were observed to increase after the combination treatment, indicating the tumors were primed to be more immune responsive. 3 out of 4 patients were reported to have stable disease after the combined therapy. The patient treated the longest started to show a tumor shrinkage at day 140 which is just at the beginning of the typical window of efficacy for immune therapy (usually between 3 and 9 months on treatment). These results are important as most patients will initially respond to therapy, but up to 75%–80% of women with advanced ovarian cancer experience tumor progression or recurrence.”
In a research update to clients today, Uddin maintained his “Speculative Buy” rating and one-year price target of $1.70 on Immunovaccine, implying a return of 39 per cent at the time of publication.
Uddin thinks the company will lose $0.08 a share on revenue of $300,000 in fiscal 2017. He thinks these numbers will improve to earnings of $0.22 on a topline of $40.0-million the following year.
Leave a Reply
You must be logged in to post a comment.





 Share
Share Tweet
Tweet Share
Share




Comment