 Nanotechnology researchers have created a new breed of tiny, glowing crystals that can detect and capture heavy-metal toxins such as lead and mercury, with soon-to-follow practical application to clean up heavy metal in contaminated water supplies.
Nanotechnology researchers have created a new breed of tiny, glowing crystals that can detect and capture heavy-metal toxins such as lead and mercury, with soon-to-follow practical application to clean up heavy metal in contaminated water supplies.
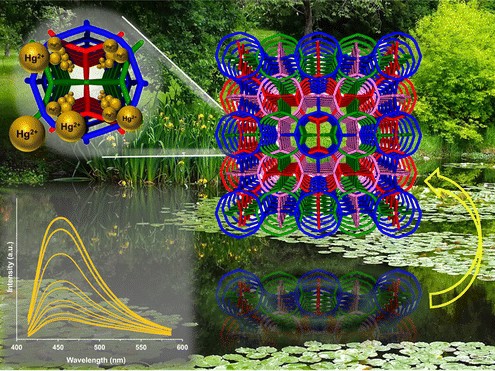
The new crystals belong to a class of materials called metal-organic frameworks or MOFs, a set of synthesized porous materials that essentially function as molecular sponges, taking in and reacting to certain molecules. First created in the 1990s, researchers have been investigating the potential use of MOFs in areas such as gas storage, catalysis and even drug delivery. In this case, the new MOFs, each about 100 microns (millions of a metre) wide, can attract and bind to heavy metals, which then triggers the whole nanoframework to glow.
“When the metal binds to the fluorescent ligand, the resulting framework fluoresces,” says Simon Teat, staff scientist at Lawrence Berkeley National Laboratory (Berkeley Lab), and co-author of the new study.
One luminescent MOF, or LMOF, that the research team tested was found to absorb more than 99 percent of the mercury from a test mixture of heavy and light metals within 30 minutes, according to recent results published in the journal Applied Materials and Interfaces. Working as both detectors and captors of heavy metal molecules is a technological advance, says Jing Li, chemistry professor at Rutgers University and lead author of the new study, who thinks the new technology could be a money-saving solution. “Others had developed MOFs for either the detection of heavy metals or for their removal, but nobody before had really investigated one that does both,” Li says.
The research team reveals that they were motivated to study the new application of MOFs by recent news of cases of heavy metal contamination in drinking water of Flint, Michigan, and Newark, New Jersey, which led them to investigate the properties of MOFs that bind to specifically to heavy metals. “With MOFs, you’re typically interested in using the holes for something,” says Teat.
The LMOFs, according to the researchers, can be used to capture toxins and can then themselves be collected, cleaned and reused for at least three cycles before their performance starts to degrade. The team hopes to test their product in a real-life scenario of actual contaminated water sources to see how well it does the job. “We would like to continue with this research,” Li said, “These are promising results, but we have a long way to go.”
MOFs have potential applications in a wide range of fields, including potential storage technologies for hydrogen gas, projected to be crucial to humanity’s future energy needs. Their novelty lies in their nanostructure, which often contains much more surface area relative to their size than naturally-occurring materials.
But contrary to previous assumptions, MOFs do exist in nature, as well. New research by scientists from Canada and Russia have come across two rare minerals first found in a coal mine in Siberia that fit the description of an MOF, in that their crystalline structure at a microscopic level has the same type of surface area to size ratio as human-made MOFs.
The discovery, published in the journal Science Advances, “completely changes the normal view of these highly popular materials as solely artificial, ‘designer’ solids,” says senior author Tomislav Friščić, associate professor of chemistry at McGill University in Montreal. “This raises the possibility that there might be other, more abundant, MOF minerals out there.”
Leave a Reply
You must be logged in to post a comment.




 Share
Share Tweet
Tweet Share
Share



Comment