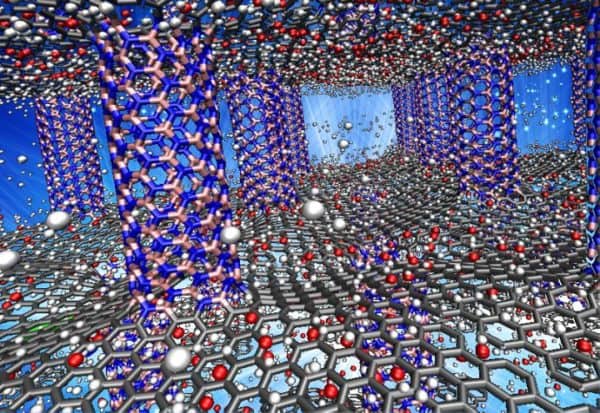
 A nanotechnology research team at Rice University in Houston, Texas, has come up with a material that may supply a solution to a tricky problem that has been plaguing alternative fuel experts for years: how to store hydrogen fuel in cars.
A nanotechnology research team at Rice University in Houston, Texas, has come up with a material that may supply a solution to a tricky problem that has been plaguing alternative fuel experts for years: how to store hydrogen fuel in cars.
As industry leaders attempt to grapple with the inevitable shift away from fossil fuel-based energy sources, hydrogen and the hydrogen-based economy that experts predict will follow need cost-effective, safe and efficient means of both producing hydrogen –electrolysis and, more commonly, a steam-reforming process using natural gas are the primary options currently on the table –and storing it.
The latter has become the challenge-du-jour in that as the lightest of the elements, hydrogen has a low energy density by volume, meaning that it’s difficult to store compressed hydrogen gas in large enough amounts to serve as a practical energy source, especially when considering relatively small and portable formats such as, say, your average mid-sized sedan. Whereas liquid hydrogen has an energy density of eight MJ/L (megajoules per litre), gasoline has four times that at 32 MJ/L, meaning that a lot of hydrogen needs to be packed into a small enough space for the hydrogen fuel cell car to be a practicable alternative to good ol’ gasoline.
But in a new study published in the journal Langmuir of the American Chemical Society, researchers have created a new computational argument for using pillared forms of boron nitride and graphene (think extremely thin sheets of carbon separated by nano-columns) as hydrogen’s new storage platform.
By the researchers’ analysis, at 2,547 square metres of surface area per gram, the composite of boron nitride and graphene held 11.6 per cent of its weight in hydrogen at room temperature and about 60 grams per litre in volumetric capacity, a result which surpasses that achieved by competing technologies for hydrogen storage like porous boron nitride and carbon nanotubes.
Researchers were able to add oxygen to the material to further attract hydrogen to its surface, making both storage and release of hydrogen more practicable through increasing and releasing pressure.
“Adding oxygen to the substrate gives us good bonding because of the nature of the charges and their interactions,” says Rice University materials scientist and study co-author, Rouzbeh Shahsavari. “Oxygen and hydrogen are known to have good chemical affinity.”
Along with storage, hydrogen fuel cell proponents face other obstacles such as the lack of current infrastructure for refueling hydrogen fueled vehicles and the still relatively large overall carbon footprint of hydrogen-sourced energy. Although the burning of hydrogen is itself an emission-neutral activity (water is the only output), sourcing hydrogen, at least by today’s methods, commonly involves methane gas in a process called steam-reforming, which makes hydrogen fuel cell vehicles less attractive in the green marketplace.
The lack of practicable hydrogen-based sources with low carbon footprints hasn’t stopped some from already jumping on the hydrogen fuel bandwagon. The City of Victoria, for instance, recently announced it would be investing in hydrogen fuel cell electric vehicles and building a refueling infrastructure in the city at a cost of between $2 and $2.5 million dollars.
Leave a Reply
You must be logged in to post a comment.


 Share
Share Tweet
Tweet Share
Share


Comment