 A non-invasive, wrist-worn blood pressure monitor developed by Kitchener-based Cloud DX has been found to be comparably accurate to an intra-arterial blood pressure catheter by an independent clinical validation study carried out at the New Brunswick Heart Centre in Saint John.
A non-invasive, wrist-worn blood pressure monitor developed by Kitchener-based Cloud DX has been found to be comparably accurate to an intra-arterial blood pressure catheter by an independent clinical validation study carried out at the New Brunswick Heart Centre in Saint John.
Cloud DX’s Pulsewave device was found to be within 5 to 10 mmHg of the direct catheter blood pressure readings, according to the study, Next-generation cloud-based blood pressure devices in chronic disease management: A direct intra-arterial pressure calibration of an oscillometric wrist cuff device for clinically reliable and accurate blood pressure measurements, conducted by Drs. Keith Brunt, PhD & Sohrab Lutchmedial, MD, FRCP(C).
The research team was motivated to determine whether the same kind of clinical decision-making typically supplied by a catheter could be supported using the Cloud DX device.
“We are pleased to see that this rigorous, independent third-party study has further validated the accuracy of our Pulsewave blood pressure monitor, and proven the flexibility of our patent-pending Cloud Diagnostics platform for measuring vital signs,” said Cloud DX CEO Robert Kaul. “Pulsewave is the first of a number of novel devices invented by Cloud Dx that are already changing the way vital signs are gathered, with an eye to a future where accurate home monitoring will be routine.”
The study was supported by the New Brunswick Health Research Foundation and Health & Life Science New Brunswick, and conducted under in accordance with Review Ethics Board Approval from the Horizon Health Network & Dalhousie Faculty of Medicine, with further support from Cardiovascular Research New Brunswick Group and the New Brunswick Heart Centre.
Dr. Brunt is Director of Innovation Development at Dalhousie University Faculty of Medicine, and Dr. Lutchmedial is the Director of Interventional Cardiology at the New Brunswick Heart Centre, and co-director of CardioVascular Research NB.
“Our study determined that innovative methods for securely gathering clinically accurate data non-invasively, including this wrist cuff device, are now mature enough for widespread adoption into new clinical trials and practices,” said Dr. Brunt. “Wrist monitors are easier to use than arm cuffs and are preferred by patients, which can improve patient compliance and accurately inform clinicians. Improving patient self-management when used at home properly, such devices will have an important part to play in 21st century medical practice to improve patient satisfaction, drug safety and reduce costs of care.”
The study concluded that “Pulsewave wrist cuff measures can be as accurate and precise as arm cuff measures in a real world patient population” and that “repeated BP measures at home, by the patient, enable clinically informed decision making; improve triage options for frail, obese and hypertensive patients, while enabling whole practice monitoring of blood pressure and assessment of drug efficacy or adverse events.”
While a paper describing the study will undergo peer-review prior to publication in early 2016, results from the study were presented on October 25, 2015 at the Canadian Hypertension Society Conference, in Mississauga ON, the Canadian Cardiovascular Congress, on October 26, 2015, in Toronto ON, and on November 3 and 4, 2015 at the 7th Annual New Brunswick Health Research Conference in Fredericton.
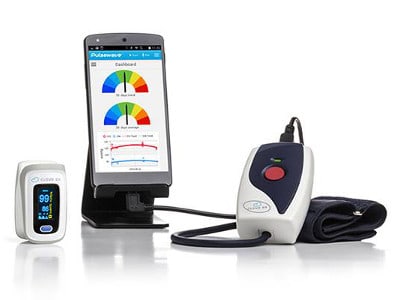
Cloud DX manufactures the FDA-cleared Pulsewave Health Monitor, a virtual medical device that records a pulse wave signal from the wrist, uploads it to its proprietary Cloud Diagnostics platform and derives medically accurate biological readings that can be securely accessed from any browser.
In September, Cloud DX won the Ontario Startup of the Year Award in the Innovation category, and took second place at the North America-wide Interface Health Challenge.
Leave a Reply
You must be logged in to post a comment.




 Share
Share Tweet
Tweet Share
Share




Comment