ProMetic Life Sciences has multi-billion dollar potential, says Paradigm
 ProMetic Life Sciences (ProMetic Life Sciences Stock Quote, Chart, News: TSX:PLI) has reached a key milestone and its idiopathic pulmonary fibrosis treatment has multi-billion dollar potential, says Paradigm Capital analyst Christopher Lam.
ProMetic Life Sciences (ProMetic Life Sciences Stock Quote, Chart, News: TSX:PLI) has reached a key milestone and its idiopathic pulmonary fibrosis treatment has multi-billion dollar potential, says Paradigm Capital analyst Christopher Lam.
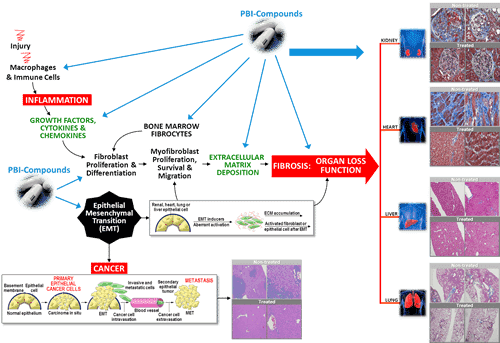
Yesterday, ProMetic announced it has had a successful preinvestigational-new-drug meeting with the United States Food and Drug Administration for its orally active anti-fibrotic lead drug candidate, PBI-4050. The company received orphan drug designation from FDA for PBI-4050 in February.
“The outcome of this meeting is that ProMetic will proceed with a placebo-controlled pivotal study in the USA in order to evaluate the combination of PBI-4050 with nintedanib or pirfenidone,” said ProMetic’s chief medical officer, Dr. John Moran. “This is an important milestone as it allows us to move forward with this multicentre study sooner than anticipated and with a clear regulatory path.”
Lam says the regulatory pathway for PBI-4050 in IPF is now established. The analyst notes that the treatment actually has three chances for success as it could be combined with Esbriet and Ofev, which are currently the only approved drugs in the market. But he thinks the most likely outcome is that ProMetic will ultimately sell or partner on PBI-4050.
“PLI has reached a critical milestone in the PBI-4050 program as the outcome of the pre-IND meeting far exceeded both our expectations and management’s,” says Lam. “Although we still view the PBI-4050 program as high risk, the new regulatory pathway provides a shorter journey and three potential roads to success. We continue to be increasingly positive on PBI-4050 and feel that investors are gaining this potentially multi-billion dollar drug for free at current levels.”
In a research update to clients today, Lam maintained his “Buy” recommendation and $3.75 one year target price on ProMetic Life Sciences, implying a return of 106% at the time of publication.
Nick Waddell
Founder of Cantech Letter
Cantech Letter founder and editor Nick Waddell has lived in five Canadian provinces and is proud of his country's often overlooked contributions to the world of science and technology. Waddell takes a regular shift on the Canadian media circuit, making appearances on CTV, CBC and BNN, and contributing to publications such as Canadian Business and Business Insider.