

Rett Syndrome breakthrough? Another play in Revive Therapeutics (Revive Therapeutics Stock Quote, Chart, News CSE: RVV) arsenal of repurposed drugs for new uses moved forward today.
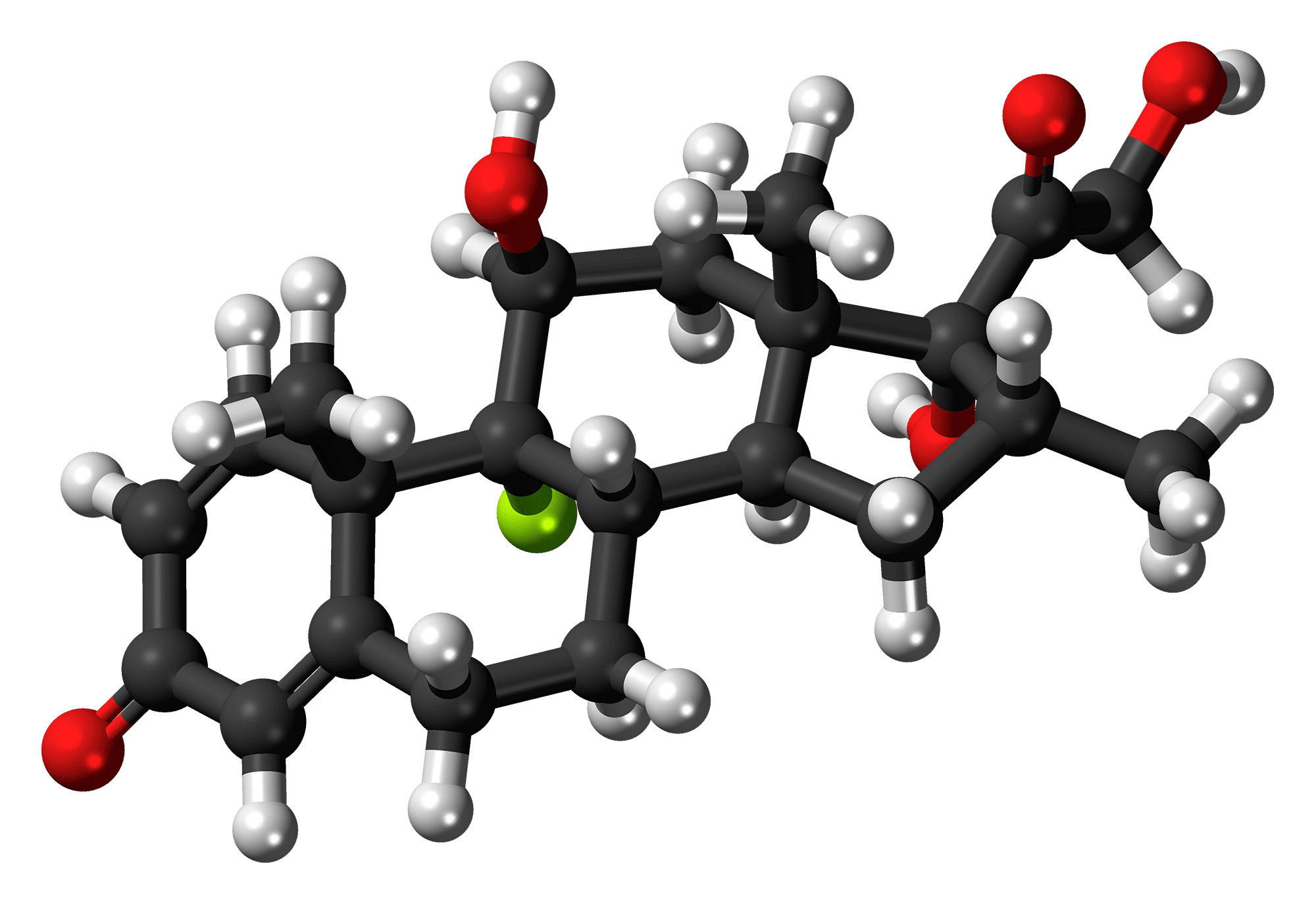
Revive reported positive results from a pre-clinical study with tianeptine for the treatment of Rett syndrome, a rare neurodevelopmental disorder.
In the United States about 16,000 people are affected by Rett, almost all are woman and children. Because the syndrome affects less than 200,00 people, it is classified as a rare disease by the National Institutes of Health.
In light of the new information, CEO Fabio Chianelli said the company will weigh its options.
“On the basis of these results and the potential neurological benefits REV-003 may have on patients with Rett syndrome, Revive is evaluating the next steps in the clinical development program, including a potential human clinical study,” he said.
Today’s news follows on the company’s late April’s request for a pre-investigational New Drug meeting with the FDA for its gout drug candidate, REV-002.
At press time, shares of Revive Therapeutics were even at $0.50.
Disclaimer: Revive Therapeutics is an annual sponsor of Cantech Letter.
More from Cantech Life Sciences
Leave a Reply
You must be logged in to post a comment.





 Share
Share Tweet
Tweet Share
Share




Comment