
Raymond James analyst Rahul Sarugaser firmly believes Alpha Cognition (Alpha Cognition Stock Quote, Chart, News, Analysts, Financials TSXV:ACOG) to be an advantageous investment option, giving the company an “Outperform 2” rating and a target price of C$3.50/share for a projected return of 483 per cent in an update to clients on Monday.
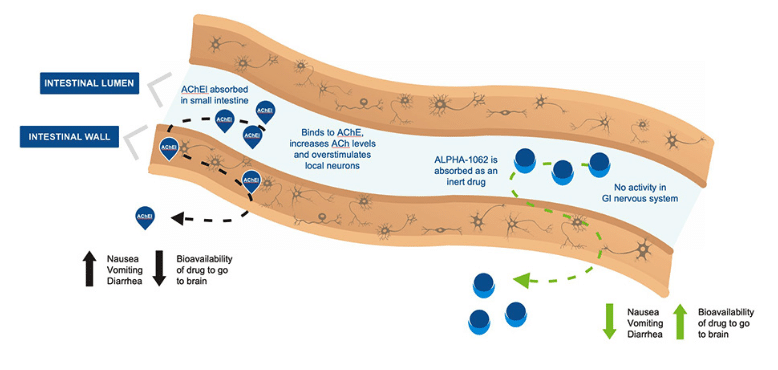
Vancouver-based Alpha Cognition is a clinical stage biopharmaceutical company dedicated to developing treatments for neurodegenerative diseases like Alzheimer’s Dementia and Lou Gehrig’s Disease, with its lead candidate being the Alpha-1062, an acetylcholinesterase inhibitor (“AChEI”) for the treatment of Alzheimer’s disease with minimal gastrointestinal side effects..
Sarugaser’s latest analysis comes with the expectation that Alpha Cognition will release top-line data for its ALPHA-1062 trial this quarter, though the company also recently indicated it would be pushing its NDA filing with the U.S. Food and Drug Administration back to 2023.
“To date, ACOG has maintained a remarkably strong record for nailing its timeline guidance, so we don’t hold a filing delay like this against the company,” Sarugaser said. “We’re impressed by ACOG’s diligence in ensuring its CMC manufacturing package is air-tight, and hence, we are confident that its commercial launch date will be unchanged. This nimbleness, to us, is a sign of this management team’s deep experience in drug development, and with the FDA.”
Following the expected release of the top-line data, the company is planning to initiate enrollment in a RESOLVE tolerability trial in the third quarter, which would pit the ALPHA-1062 candidate against a placebo with a data readout expected in mid-2023. According to Sarugaser, Alpha Cognition intends to use the RESOLVE readout as an accelerator of adoption and as a tool to petition the FDA to augment ALPHA-1062’s label.
The delay on its NDA filing with the FDA pushes it back to the second quarter of 2023 on the timeline, with the delay coming on account of the company wanting to ensure maximum compliance with the FDA’s guidelines on chemical manufacturing and controls (CMC).
According to Sarugaser, the FDA has been heavily focused on manufacturing as of late, with no less than nine companies having been issued Complete Response Letters (CRL) due to deficiencies in its CMC.
Alpha Cognition has been working closely with the FDA on its trials to ensure smooth progress through the regulatory apparatus, with the FDA recommending that the company do additional stability work on ALPHA-1062’s highest formulation, with that work on lower does having already been completed.
“In the company’s view, the strongest possible drug launch would benefit from all possible dose levels being included in the label (rather than included on the label as a supplement), so ACOG decided to listen to the FDA and undertake this work ahead of its NDA filing,” Sarugaser said.
From there, Alpha Cognition is estimating FDA marketing clearance on its offering to come in the final quarter of 2023, paving the way for an official marketing launch in the opening quarter of 2024.
The news came out of the company’s first quarter financial results for the 2022 fiscal year, which were released on May 31 and included research and development expenses of $1.8 million (all dollar amounts from the company’s report are in US dollars), along with general and administrative expenses of $0.7 million.
Alpha Cognition had $9.1 million in cash and equivalents available as of March 31, paired with a reported loss of $2.7 million.
“Alpha Cognition reported positive preclinical data with ALPHA-1062 intranasal for mTBI and ALPHA-0602 for ALS during the first quarter of 2022 and continued to build-out our experienced leadership team. The Company also plans to meet with the FDA for the ALPHA-1062 intranasal for mTBI during the third quarter of 2022,” said Michael McFadden, Chief Executive Officer of Alpha Cognition in the release.
With the pushback on its timeline, Sarugaser has deferred his revenue forecast, as he no longer projects $17 million in revenue for 2023, which would have been the first quarter of meaningful revenue generation. Meanwhile, he also forecasts an adjusted EBITDA loss of $7 million in 2022, before a positive turn of $7 million projected in 2023.
Overall, Sarugaser believes Alpha Cognition has made a smart decision in recalibrating its timeline in hopes of a smoother approval process.
“With several recent examples of CMC-related setbacks for drug developers and a perceived attitude of high-caution coming from the FDA’s neuro division post-Aduhelm, we think ACOG’s maneuvers here are quite wise and, if all goes to plan, will have limited or no impact on ALPHA-1062’s launch date: 1Q24,” Sarugaser said.
Alpha Cognition’s share price has fallen by 46.8 per cent over the course of the year, peaking early at $1.13/share on January 18. The stock closed Monday trading at $0.58/share, representing its lowest point of 2022.




 Share
Share Tweet
Tweet Share
Share




Comment