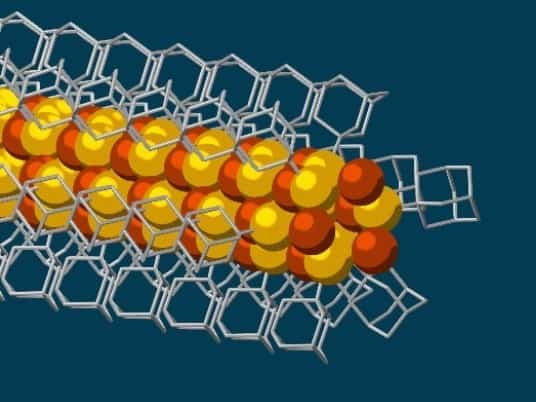
 Researchers at Stanford University and the U.S. Department of Energy have made the thinnest possible electrically conductive wires, only three atoms thick, using diamondoids.
Researchers at Stanford University and the U.S. Department of Energy have made the thinnest possible electrically conductive wires, only three atoms thick, using diamondoids.
The wires with a semi-conducting core of copper and sulfur are made with the help of diamondoids, hydrocarbon molecules in which the carbon atoms are arranged in a lattice similar to that found in crystalline diamond. The new results have a range of potential applications in nanotechnology and electronics, say the study’s authors.
“What we have shown here is that we can make tiny, conductive wires of the smallest possible size that essentially assemble themselves,” says Hao Yan, postdoctoral researcher at Stanford’s Institute for Materials and Energy Sciences and lead author of the study published in the journal Nature Materials. “The process is a simple, one-pot synthesis. You dump the ingredients together and you can get results in half an hour. It’s almost as if the diamondoids know where they want to go.”
Diamondoids exhibit strong van der Waals forces of attraction, causing the tiny, cage-like structures to link up together into larger crystals. The new results stem from the insertion of sulfur atoms into each diamond structure, only 10 carbon atoms in total, so that the cage then bonds in solution with individual copper ions, creating a conductive core surrounded by the insulating diamondoid lattice. The whole structure naturally links together to form strands of nanowires, say the study’s authors.
“Much like LEGO blocks, they only fit together in certain ways that are determined by their size and shape,” said Stanford graduate student and study co-author Fei Hua Li. “The copper and sulfur atoms of each building block wound up in the middle, forming the conductive core of the wire, and the bulkier diamondoids wound up on the outside, forming the insulating shell.”
Nanowires differ from other conducting wires in more than just size. Due to the application of quantum mechanics, electrons moving through nanowires actually behave differently, exhibiting properties such as electron tunnelling, where electrons are able to transport across an insulator without actually going “through” it, and, important for electrical conductivity, electrons in nanowires can travel along a circuit without colliding with nearby atoms – and thus without generating the heat normally produced in an electrical current.
Scientists see these properties as giving nanowires unique advantages. Work by researchers at the University of British Columbia in Vancouver and MIT in Cambridge, Massachusetts, has revealed, for example, that nanowires can be threaded together to create supercapacitors able to deliver short bursts of high power, with potential uses in a range of health-monitoring and wearable technologies.
“You can imagine weaving those into fabrics to generate energy,” says Nicholas Melosh of the SLAC National Accelerator Laboratory with the U.S. Department of Energy and co-author of the new study. “This method gives us a versatile toolkit where we can tinker with a number of ingredients and experimental conditions to create new materials with finely tuned electronic properties and interesting physics.”
Leave a Reply
You must be logged in to post a comment.



 Share
Share Tweet
Tweet Share
Share




Comment