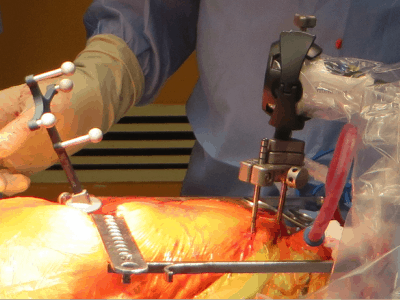
 Waterloo’s Intellijoint Surgical Inc. has been awarded US Food and Drug Administration clearance for the next generation of its intellijoint HIP, designed to help orthopaedic surgeons better establish cup placement in the patient, adjust leg length and joint rotation.
Waterloo’s Intellijoint Surgical Inc. has been awarded US Food and Drug Administration clearance for the next generation of its intellijoint HIP, designed to help orthopaedic surgeons better establish cup placement in the patient, adjust leg length and joint rotation.
The next-generation Intellijoint HIP launched in Canada in July at the MaRS Discovery District in Toronto.
“With the previous generation of the product, our use showed improved accuracy and precision of implant selection without a significant expense or impact on OR time,” said Dr. Wayne Paprosky, professor and widely published orthopaedic surgeon at Rush Medical Center in Chicago. “The new generation of intellijoint HIP provides surgeons with additional critical implant alignment measurements including cup inclination and anteversion, which will be a potential game changer of how hip surgery is performed in the US.”
The intellijoint HIP is a smart surgical instrument that allows orthopaedic surgeons to control several key measurements to ensure a better outcome for hip replacement patients.
The HIP is an example of miniature 3D surgical measurement technology in the sterile field.
Being cost-effective and compatible with the majority of implant vendors, it adds a new possibility in computer-assisted orthopaedic surgery.
For example, 62% of patient legs are lengthened up to 1 cm after surgery. Half of patients will perceive this discrepancy for 12 months after surgery, resulting in 30% dissatisfaction for five to eight years after the procedure.
Up to 78% of patients have been involved in a post-op legal dispute with surgeons, with leg length discrepancy being one of the most frequent complaints.
“Intellijoint was founded in 2010 with the vision of developing and commercializing an innovative technology that is capable of providing real time 3D surgical measurement data, without adding significant time, expense or complexity to surgery,” says Armen Bakirtzian, Chief Executive Officer and Co-founder of Intellijoint Surgical. “Now, with FDA clearance of intellijoint HIP, we’ve realized this vision and are ready to bring its benefits to the largest market in the world.”
Intellijoint operated for four years in stealth mode, being used in over 200 live surgeries in Toronto, Waterloo, Chicago, New York and Anaheim.
Leave a Reply
You must be logged in to post a comment.





 Share
Share Tweet
Tweet Share
Share




Comment