
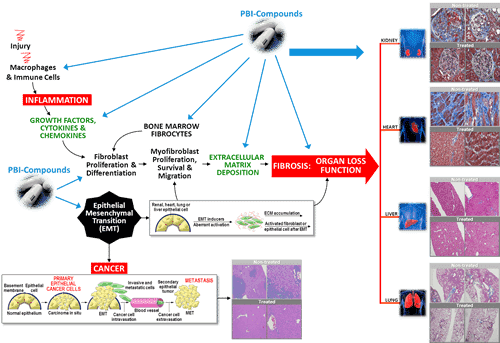
At the recent 2013 European Respiratory Society Annual Congress, Prometic released new preclinical data that showed PBI-4050 significantly reduced the tissue scarring in the lungs of its animal trial subjects. Prometic Life Sciences (TSX:PLI) is fast becoming one of the most talked about biotech success stories on the TSX this year.
Shares of Prometic have caught fire in the latter half of 2013, rising from barely thirty cents in July to recent highs over $.90.
The reason? Investors are increasingly optimistic that PBI-4050, Prometic’s new therapeutic aimed at idiopathic pulmonary fibrosis, is the real deal. Prometic, which is now entering clinical trial stages with the treatment, released new preclinical data at the 2013 European Respiratory Society Annual Congress held in Barcelona last month that showed it significantly reduced the tissue scarring in the lungs of its animal trial subjects.
What’s more, the company noted that when combined with Pirfenidone, which is currently the only commercially approved product for the treatment of idiopathic pulmonary fibrosis, the treatment “generated unprecedented reduction of fibrosis resulting in a significant improvement of organ function”.
Idiopathic pulmonary fibrosis is a chronic and fatal disease characterized by a progressive decline in lung function. The disease affects 130,000 people each year in the United States alone, killing 40,000 of them, the same number as breast cancer.
Prometic’s share price began its rise from the bargain bin last October, when China-based Shenzhen Hepalink Pharmaceutical took a 10% stake with a $10-million equity investment in the company. It made further gains in January when it received confirmation from partner Octapharma of the regulatory approval of Octaplas by the FDA for the U.S. market. Octaplas, a treatment for patients with blood clotting disorders, has already treated more than two-million patients outside the United States, and incorporates ProMetic’s PrioClear into its manufacturing process.
Founded in 1992, Laval-based ProMetic Life Sciences designs technology that is used to remove pathogens from blood, and extract and recover proteins from plasma. The company has a number of therapeutics and protein technologies that target everything from Chemotherapy-induced anemia, to Cancer related anemia, to its Prion Capture Technology, which enhances detection of “mad cow disease” in cattle.
___________
Leave a Reply
You must be logged in to post a comment.




 Share
Share Tweet
Tweet Share
Share




Comment